Question: Given the reaction and data below, calculate the equilibrium constant at 310 K and identify if the reaction is RF (reactant-favored) or PF (product-favored)
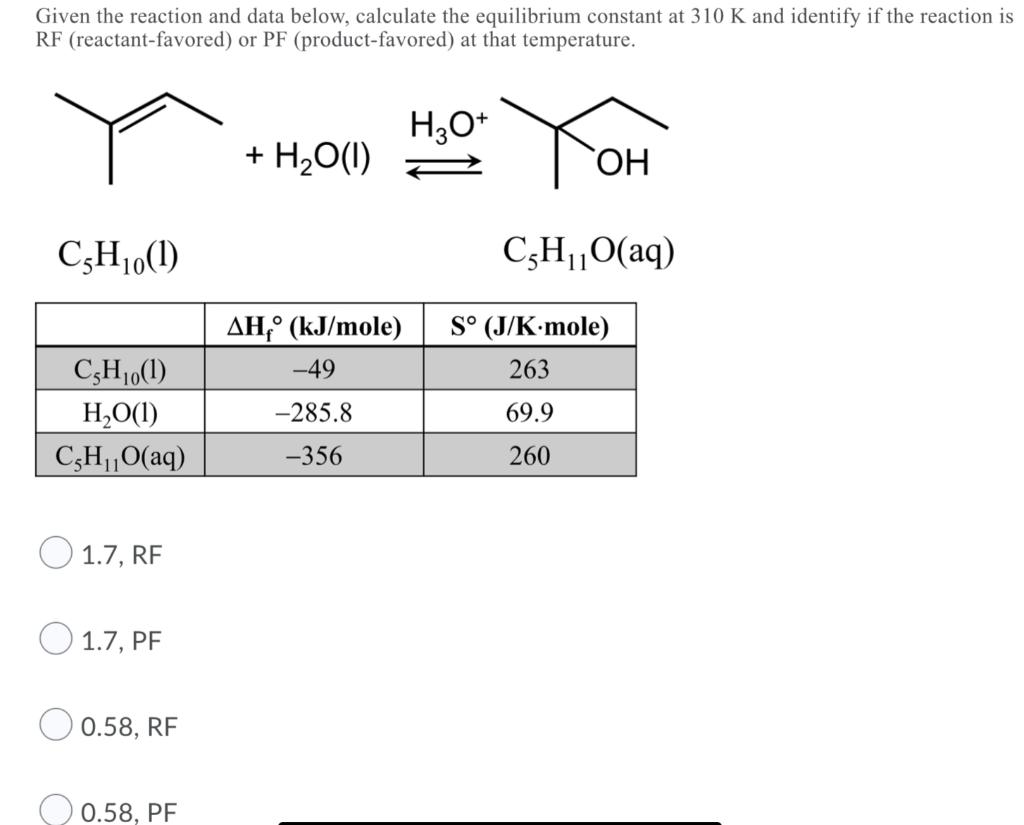
Given the reaction and data below, calculate the equilibrium constant at 310 K and identify if the reaction is RF (reactant-favored) or PF (product-favored) at that temperature. H3O+ C5H0(1) C,H0(1) HO(1) C5HO(aq) 1.7, RF 1.7, PF 0.58, RF 0.58, PF + HO(1) AH, (kJ/mole) -49 -285.8 -356 YOH OH C5HO(aq) 11 S (J/K-mole) 263 69.9 260
Step by Step Solution
3.49 Rating (152 Votes )
There are 3 Steps involved in it
2 C5H10 H0 KJm... View full answer

Get step-by-step solutions from verified subject matter experts


