Question: Given the reaction below, answer the following questions: A) Predict the effect of a nitro substituent in para position on the rate of the reaction.
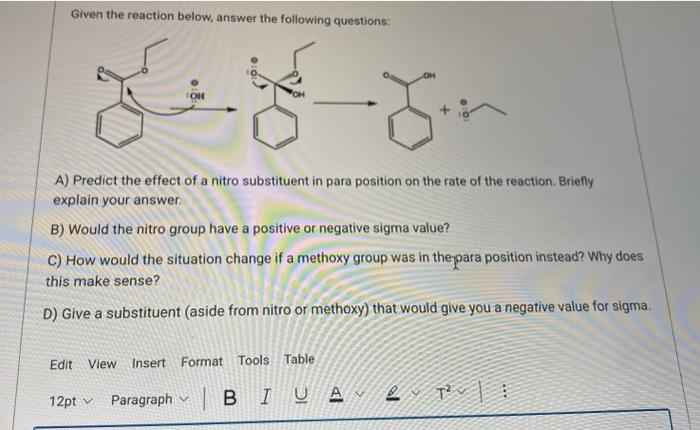
Given the reaction below, answer the following questions: A) Predict the effect of a nitro substituent in para position on the rate of the reaction. Briefly explain your answer. B) Would the nitro group have a positive or negative sigma value? C) How would the situation change if a methoxy group was in theypara position instead? Why does this make sense? ) Give a substituent (aside from nitro or methoxy) that would give you a negative value for sigma
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


