Question: Given the substances: C2H5FC2H5OHC3H8 Determine which has the highest boiling point and which has the next highest boiling point, and choose the correct explanations for
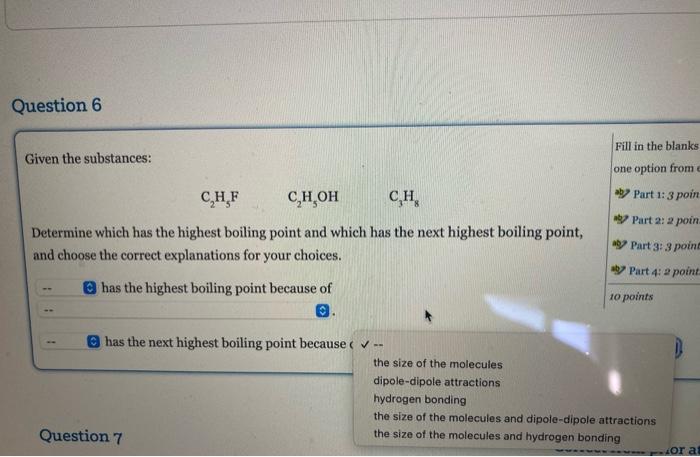
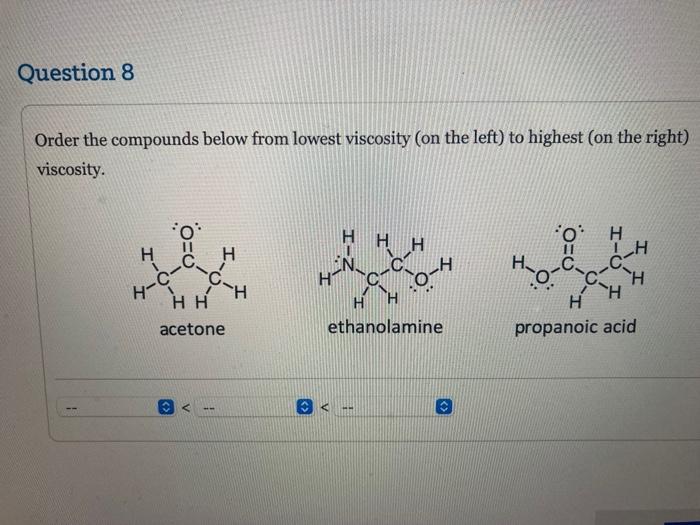
Given the substances: C2H5FC2H5OHC3H8 Determine which has the highest boiling point and which has the next highest boiling point, and choose the correct explanations for your choices. has the highest boiling point because of has the next highest boiling point because (. the size of the molecules dipole-dipole attractions hydrogen bonding the size of the molecules and dipole-dipole attractions Question 7 the size of the molecules and hydrogen bonding Order the compounds below from lowest viscosity (on the left) to highest (on the right) viscosity. ethanolamine propanoic acid
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


