Question: Given the thiocyanate ion, [SCN] , determine: (a) all possible Lewis structures; (b) the preferred structure using formal charges; (c) the number of electron domains;
![Given the thiocyanate ion, [SCN] , determine: (a) all possible Lewis](https://dsd5zvtm8ll6.cloudfront.net/si.experts.images/questions/2024/09/66f9755578f18_53366f9755514341.jpg)
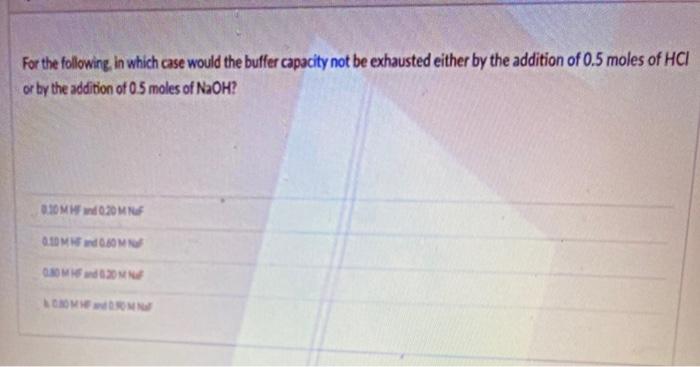
Given the thiocyanate ion, [SCN] , determine: (a) all possible Lewis structures; (b) the preferred structure using formal charges; (c) the number of electron domains; (d) the electron domain geometry; (e) the molecular geometry : (f) if the molecule is polar or non-polar; and (g) the hybridization of the central atom. For the following in which case would the buffer capacity not be exhausted either by the addition of 0.5 moles of HCI or by the addition of 0.5 moles of NaOH? OM 20MM 0.100.80 MOON COM Given the thiocyanate ion, [SCN] , determine: (a) all possible Lewis structures; (b) the preferred structure using formal charges; (c) the number of electron domains; (d) the electron domain geometry; (e) the molecular geometry : (f) if the molecule is polar or non-polar; and (g) the hybridization of the central atom. For the following in which case would the buffer capacity not be exhausted either by the addition of 0.5 moles of HCI or by the addition of 0.5 moles of NaOH? OM 20MM 0.100.80 MOON COM
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


