Question: Given the TLC (thin layer chromatography) plate below determine the compound(s) present within the unknown. - The following TLC plate has been fully developed and

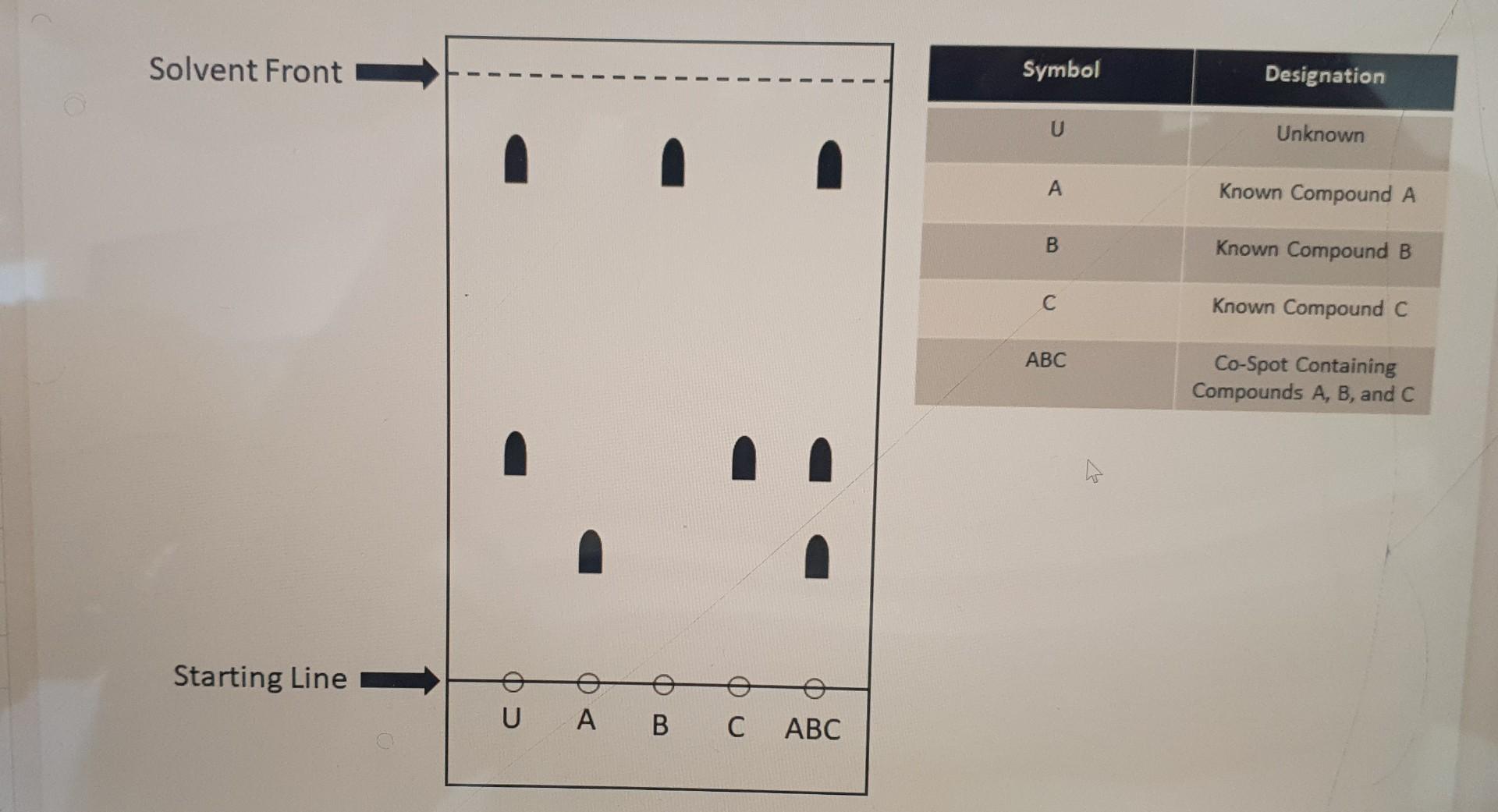

Given the TLC (thin layer chromatography) plate below determine the compound(s) present within the unknown. - The following TLC plate has been fully developed and marked so that all spots are indicated. - The stationary phase of the TLC plate is composed of relatively polar silica gel. In other words, a standard stationary phase is being used. - The "starting line" represents where a given compound or multiple compounds have been spotted before development of the TLC plate (see key). - The "solvent front" represents the distance traveled by the eluent (solvent) up the TLC plate. (Multiple Answer: Multiple answers may be correct, and all correct answers must be indicated for full credit) \begin{tabular}{|c|c|} \hline Symbol & Designation \\ \hline U & Unknown \\ \hline A & Known Compound A \\ \hline B & Known Compound B \\ \hline C & Known Compound C \\ \hline ABC & \begin{tabular}{c} Co-Spot Containing \\ Compounds A, B, and C \\ \hline \end{tabular} \\ \hline \end{tabular} Compound A Compound B Compound C
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


