Question: Goal Solve for the efficiency of a heat engine using a five - step process the includes: Making a state table. Making a process table.
Goal Solve for the efficiency of a heat engine using a fivestep process the includes:
Making a state table.
Making a process table.
Calculating the totals for Work, Heat, and InternalEnergyChange.
Identifying the heat input hot reservoir and output cold reservoir
Calculating the efficiency of the engine.
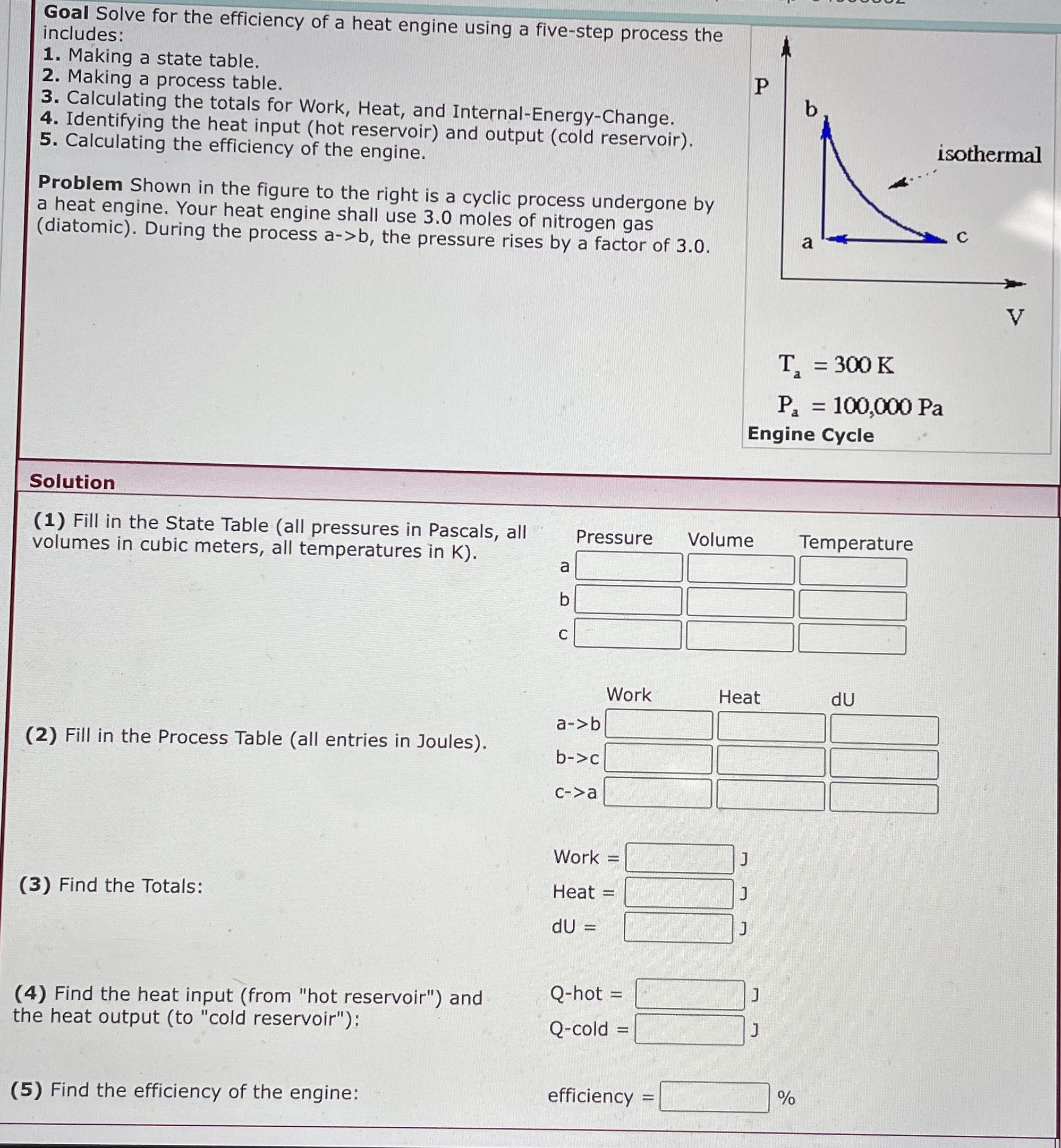
Problem Shown in the figure to the right is a cyclic process undergone by a heat engine. Your heat engine shall use moles of nitrogen gas diatomic During the process the pressure rises by a factor of
Engine Cycle
Solution
Fill in the State Table all pressures in Pascals, all volumes in cubic meters, all temperatures in K
Fill in the Process Table all entries in Joules
Find the Totals:
Find the heat input from "hot reservoir" and the heat output to "cold reservoir":
tablePressure Volume Temperature,abc
Work J
Heat J
hot
cold
efficiency
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


