Question: google for the table if needed. Using data from Table A-25, calculate the equilibrium constant expressed as logio , for the water-gas shift reaction CO
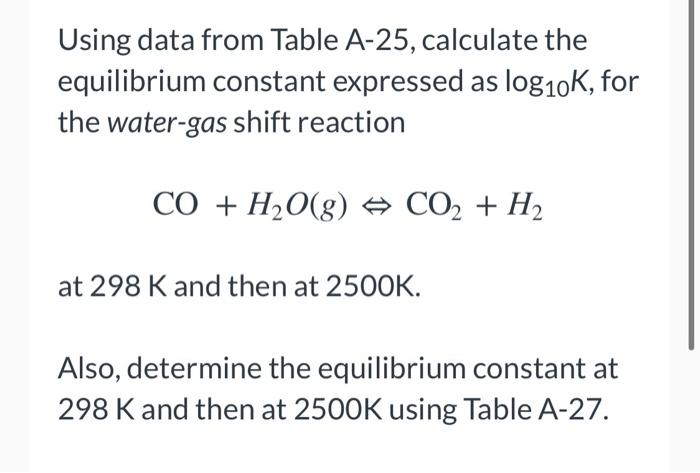
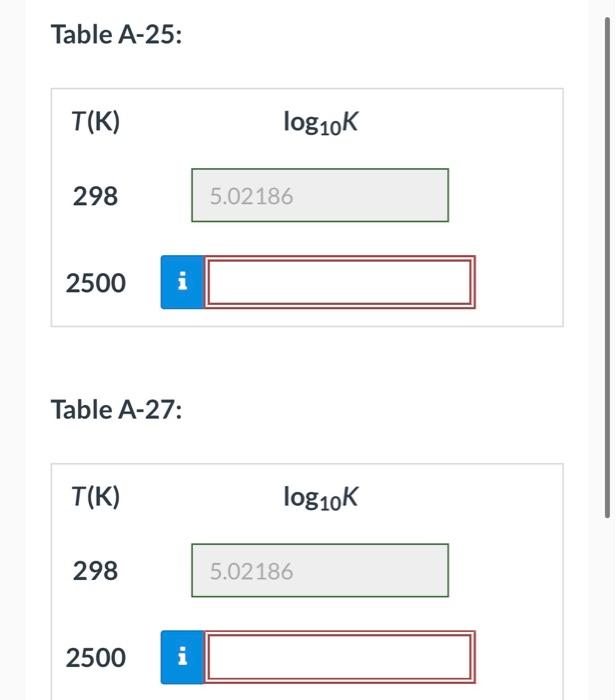
Using data from Table A-25, calculate the equilibrium constant expressed as logio , for the water-gas shift reaction CO + H2O(g) o CO2 + H2 at 298 K and then at 2500K. Also, determine the equilibrium constant at 298 K and then at 2500K using Table A-27. Table A-25: T(K) log10K 298 5.02186 2500 IN Table A-27: T(K) log10K 298 5.02186 2500
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


