Question: H 2 ( g ) + I 2 ( g ) 2HI( g ) at 150C with the reaction vessel being charged in such a
 H2(g) + I2(g) 2HI(g) at 150C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm. Refer to Exhibit 14-2. What is the value of the equilibrium constant at this temperature?
H2(g) + I2(g) 2HI(g) at 150C with the reaction vessel being charged in such a way that the initial partial pressure of all the species in the reaction (both reactants and products) is 0.200 atm. Refer to Exhibit 14-2. What is the value of the equilibrium constant at this temperature?
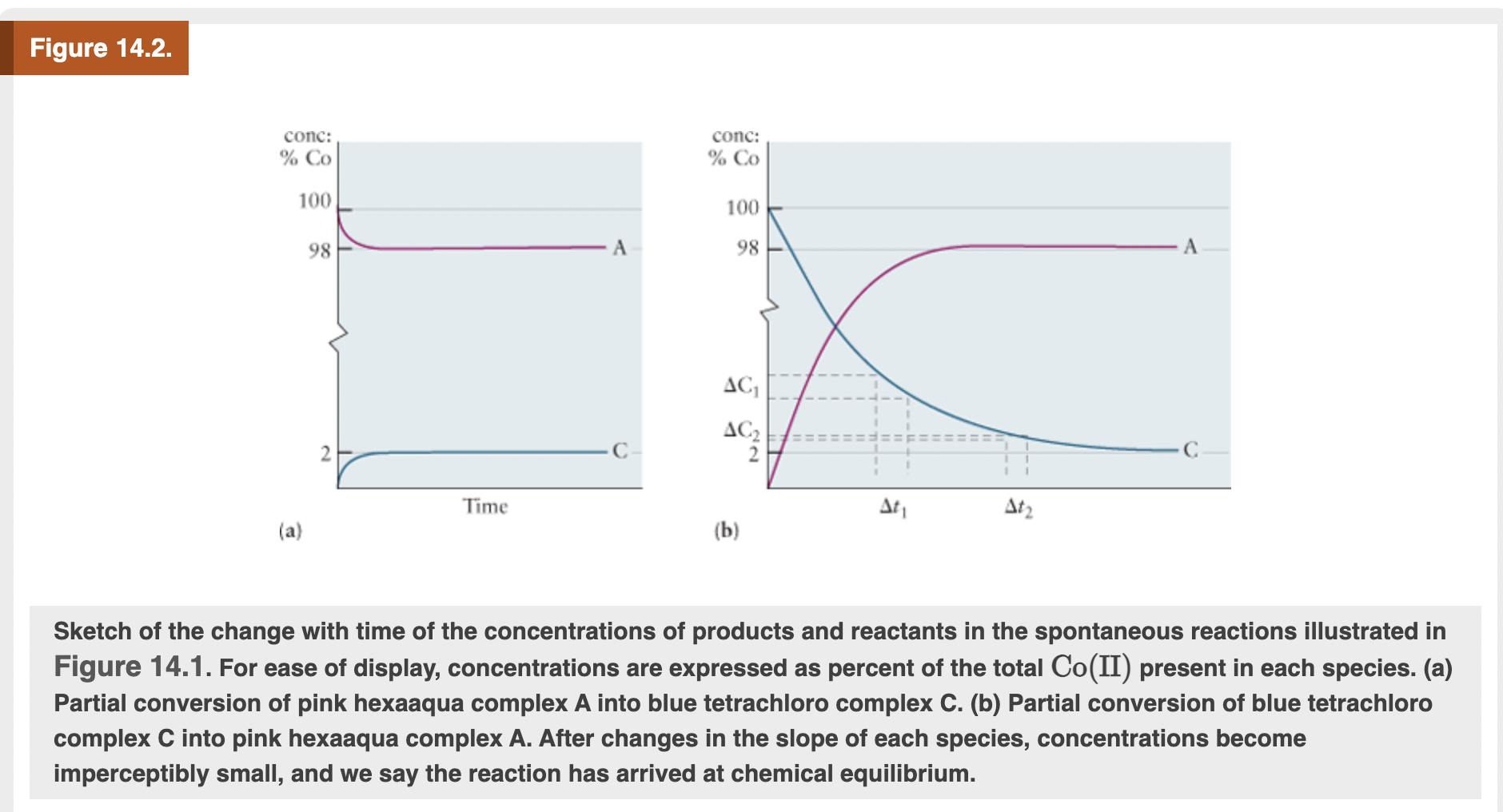
Sketch of the change with time of the concentrations of products and reactants in the spontaneous reactions illustrated in Figure 14.1. For ease of display, concentrations are expressed as percent of the total Co(II) present in each species. (a) Partial conversion of pink hexaaqua complex A into blue tetrachloro complex C. (b) Partial conversion of blue tetrachloro complex C into pink hexaaqua complex A. After changes in the slope of each species, concentrations become imperceptibly small, and we say the reaction has arrived at chemical equilibrium
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


