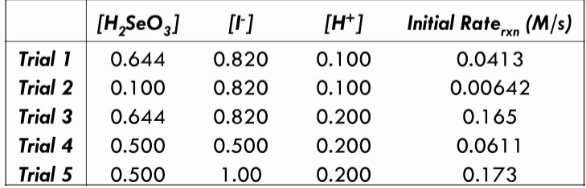
Question: H 2 SeO 3 + 6I - + 4H + --> Se + 2I 3 - + 3H 2 O Determine the experimental rate law
H2SeO3 + 6I- + 4H+ --> Se + 2I3- + 3H2O
Determine the experimental rate law and rate constant (k) for the reaction. Find the order for all 3 reactants.
\begin{tabular}{l|cccc} & {[H2SeO3]} & {[L]} & {[H+]} & Initial Rate rxn(M/s) \\ \hline Trial 1 & 0.644 & 0.820 & 0.100 & 0.0413 \\ Trial 2 & 0.100 & 0.820 & 0.100 & 0.00642 \\ Trial 3 & 0.644 & 0.820 & 0.200 & 0.165 \\ Trial 4 & 0.500 & 0.500 & 0.200 & 0.0611 \\ Trial 5 & 0.500 & 1.00 & 0.200 & 0.173 \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


