Question: (h) Consider the binary phase diagram below. (20) i. What is the eutectic composition and temperature? ii. At 150C, what is the solubility limit of
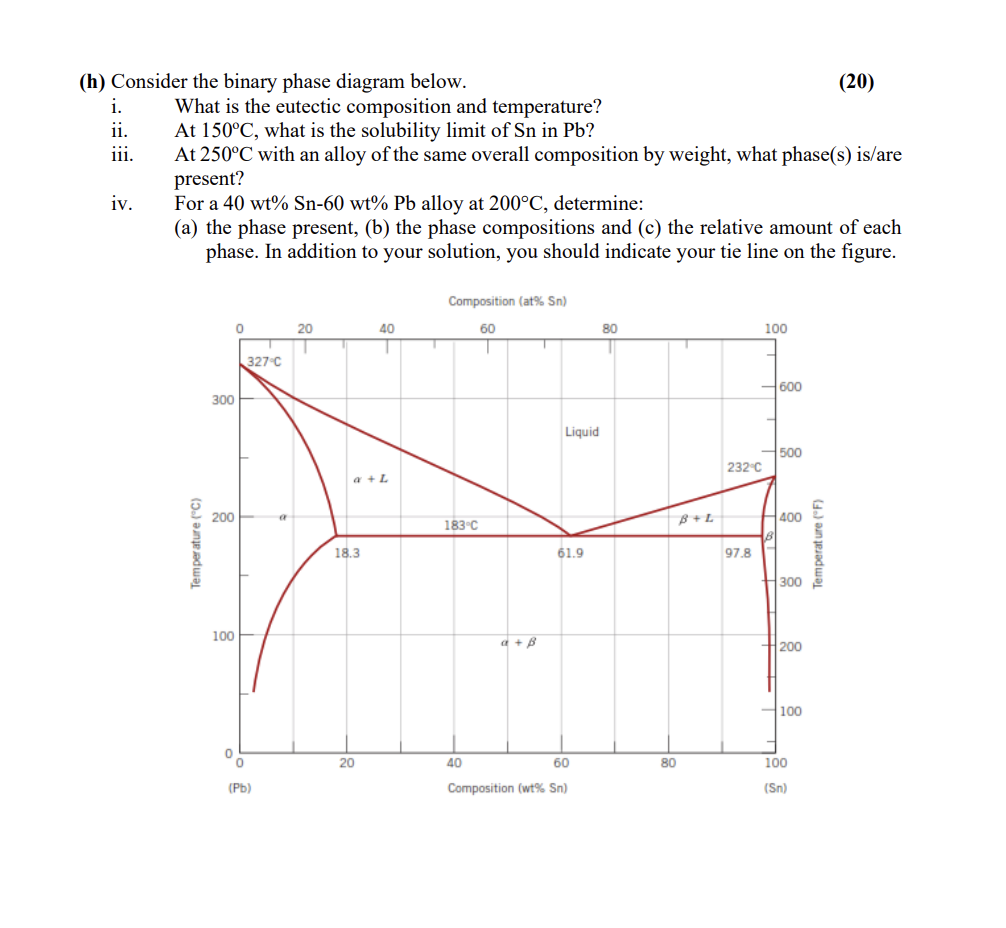
(h) Consider the binary phase diagram below. (20) i. What is the eutectic composition and temperature? ii. At 150C, what is the solubility limit of Sn in Pb? iii. At 250C with an alloy of the same overall composition by weight, what phase(s) is/are present? iv. For a 40 wt% Sn-60 wt% Pb alloy at 200C, determine: (a) the phase present, (b) the phase compositions and (c) the relative amount of each phase. In addition to your solution, you should indicate your tie line on the figure. Composition (at% Sn) 0 20 60 80 100 40 1 T 327C 600 300 Liquid 500 232.c a + L 200 183c BL 400 Temperature (C) 18.3 Temperature (F) 61.9 97.8 300 100 + B 200 100 0 0 20 40 60 80 100 (Pb) Composition (wt% Sn) (Sn)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


