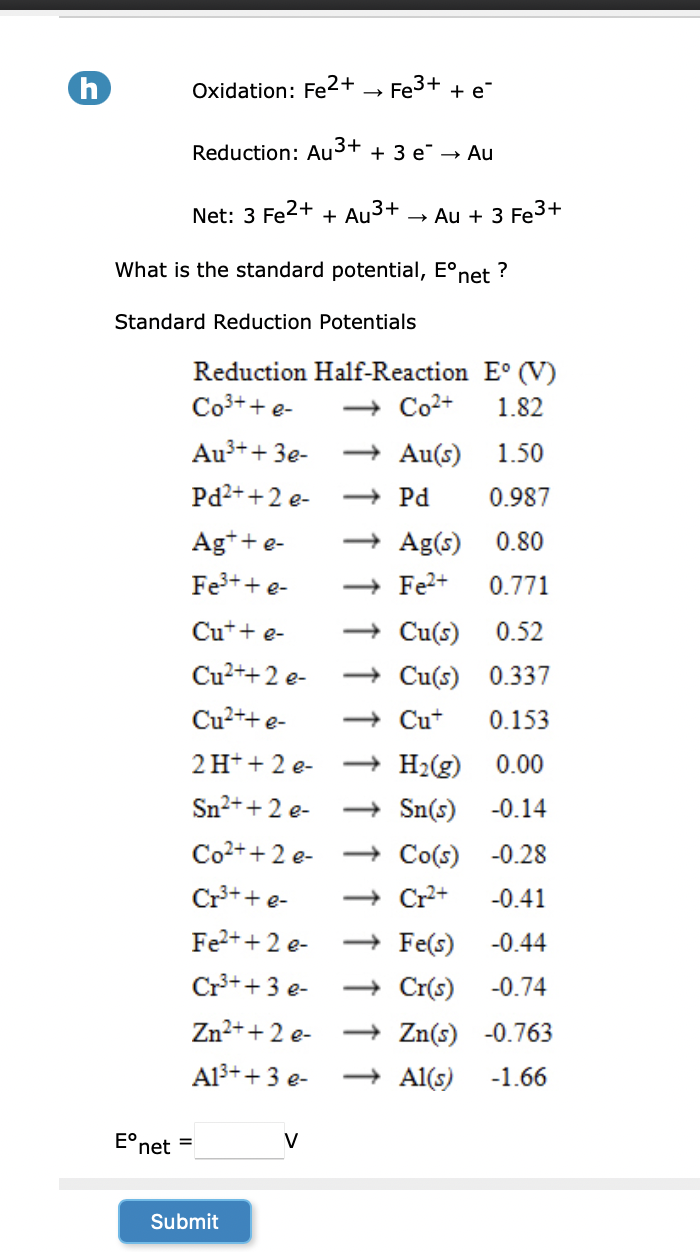
Question: ( h ) Oxidation: F e 2 + F e 3 + + e - Reduction: A u 3 + + 3 e - A
h Oxidation:
Reduction:
Net:
What is the standard potential, net
Standard Reduction Potentials
Reduction HalfReaction
longrightarrowPd,
longrightarrowAg
longrightarrowCu
longrightarrowSn
longrightarrowCo
longrightarrowFe
longrightarrowCr
longrightarrowZn
net
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


