Question: a. Convert the following Newman projection of compound G to a three dimensional line structure in the given conformation. Place the aldehyde functional group
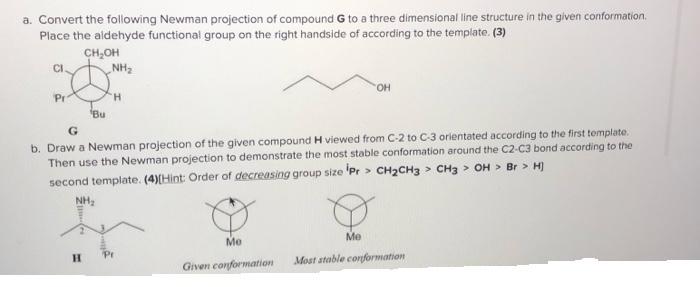
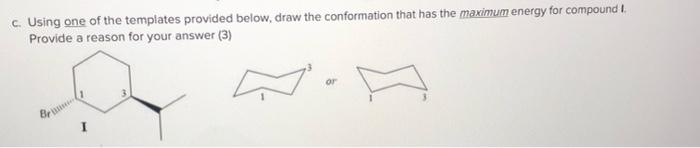
a. Convert the following Newman projection of compound G to a three dimensional line structure in the given conformation. Place the aldehyde functional group on the right handside of according to the template. (3) CH,OH CI. NH2 OH Pr H. Bu G b. Draw a Newman projection of the given compound H viewed from C-2 to C-3 orientated according to the first template. Then use the Newman projection to demonstrate the most stable conformation around the C2-C3 bond according to the second template. (4)(Hint: Order of decreasing group size 'Pr > CH2CH3 > CH3 > OH > Br > H) NH, Me Me H. Pr Given conformation Most atable conformation c. Using one of the templates provided below, draw the conformation that has the maximum energy for compound I. Provide a reason for your answer (3) or
Step by Step Solution
3.35 Rating (155 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


