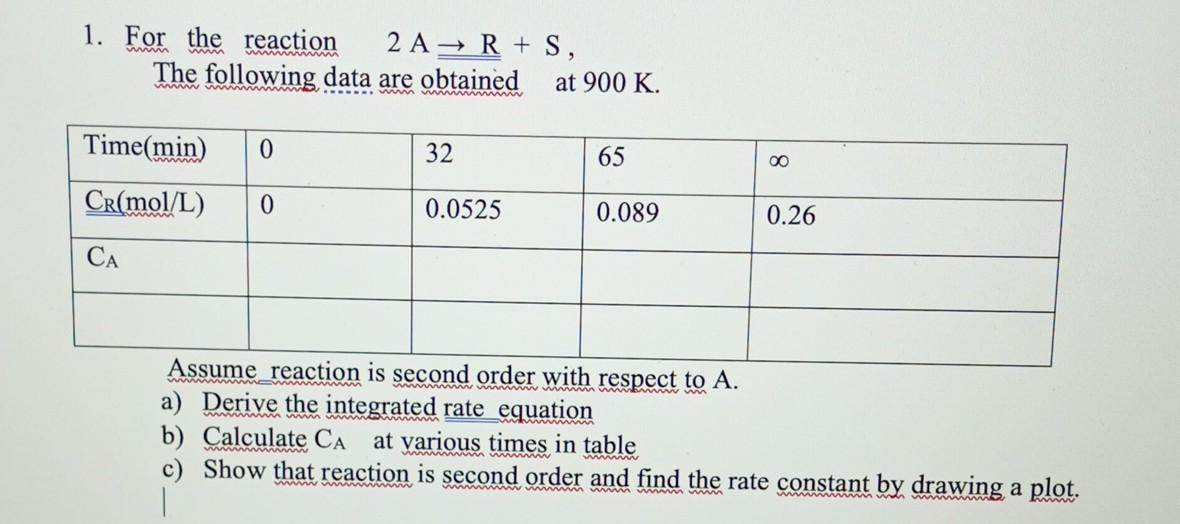
Question: hand calculation pls i will vote high 1. For the reaction 2AR+S, The following data are obtained at 900K. Assume reaction is second order with
hand calculation pls i will vote high
1. For the reaction 2AR+S, The following data are obtained at 900K. Assume reaction is second order with respect to A. a) Derive the integrated rate equation b) Calculate CA at various times in table c) Show that reaction is second order and find the rate constant by drawing a plot
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


