Question: Have no clue how to do Normality INTERACTIVE EXAMPLE Calculating Various Methods of Solution Composition from the Molarity A 2.71M solution of citric acid (H3C6H5O7)
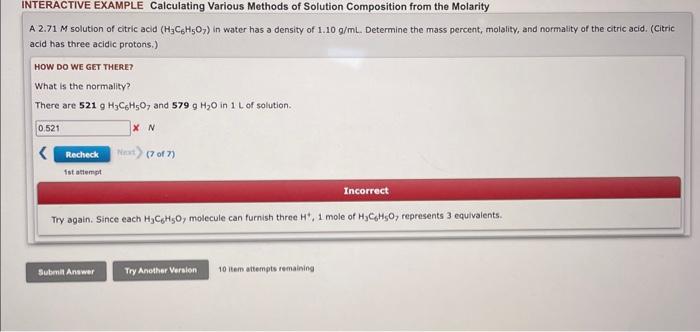
INTERACTIVE EXAMPLE Calculating Various Methods of Solution Composition from the Molarity A 2.71M solution of citric acid (H3C6H5O7) in water has a density of 1.10g/mL. Determine the mass percent, molality, and normality of the citric acid. (Citric acid has three acidic protons.) HOW DO WE GET THERE? What is the normality? There are 521gH3C6H5O7 and 579gH2O in 1L of solution. N (7 of n fst atemint Incorrect Try again. Since each H3C6H5O, molecule can furnish three H+,1 mole of H3C6H5O7, represents 3 equivalents
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


