Question: Having issues with how to solve the math The following reaction has an equilibrium constant of 50 . at a given temperature. I2(g)+H2(g)2HI(g) If you

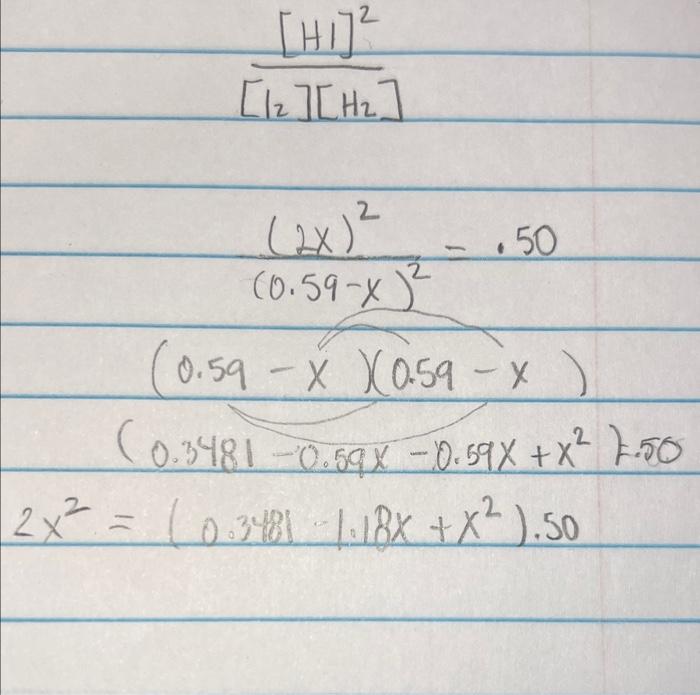
The following reaction has an equilibrium constant of 50 . at a given temperature. I2(g)+H2(g)2HI(g) If you have 0.59 moles of I2(g) and 0.59 moles of H2(g) in a 1.0L container initially, how many moles of H2 will be present when the system reaches equilibrium? 0.14 moles 0.02 moles 0.13 moles 0.07 moles [H2][H2][HI]2 (0.59x)2(2x)2=..50 (0.59x)(0.59x) (0.34810.59x0.59x+x2)=50 2x2=(0.34811.18x+x2).50
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


