Question: heat (q) gained by the solution can bo calculated deveview I q=CEmT Where Cs is the specific heat, m is the mass of the solution,
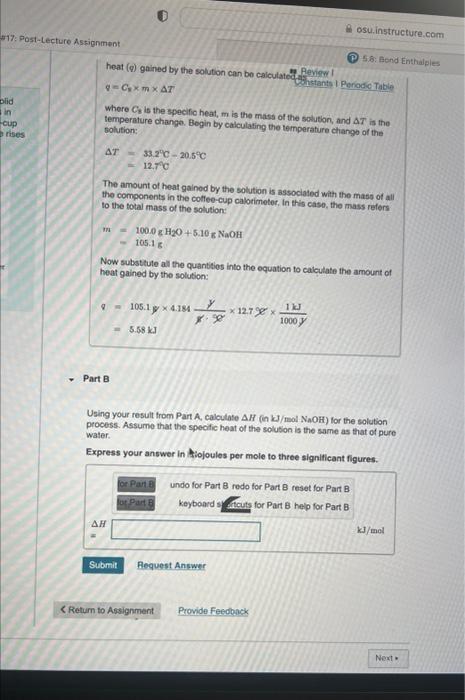
heat (q) gained by the solution can bo calculated deveview I q=CEmT Where Cs is the specific heat, m is the mass of the solution, and T is the. temperature change. Begin by calculating the temperature chango of the solution: T=33.2C20.5C=12.7C The amount of heat gained by the solution is assoclated with the mass of all the components in the cotfee-cup calorimeter, In this caso, the mass refers to the total mass of the solution: m=100.0gH2O+5.10NNaOH=105.1g Now substtute all the quantitios into the equation to calculate the amount of heat gained by the solution: q=105.1y4.184g9gy12.7981000J1kJ=5.58kJ Part B Using your result from Pain A, calculate H(inkJ/molNaOH) for the solution process. Assume that the specific heat of the solution is the same as that of pure water. Express your answer in hiojoules per mole to three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


