Question: Heating and Cooling Curves Consider heating solid water (ice) until it becomes liquid and then gas (steam) (Figure 1). Alternatively, consider the reverse process,
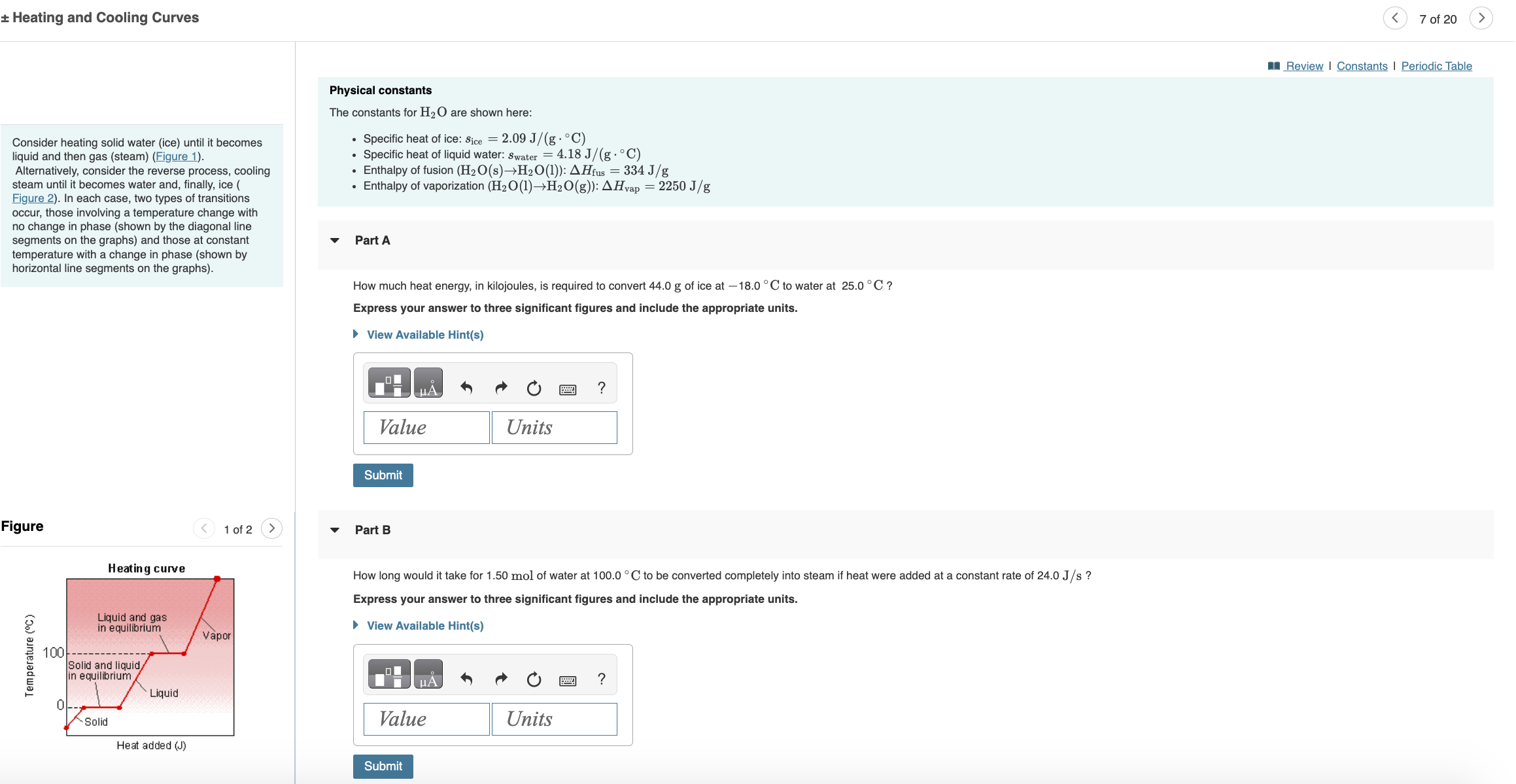
Heating and Cooling Curves Consider heating solid water (ice) until it becomes liquid and then gas (steam) (Figure 1). Alternatively, consider the reverse process, cooling steam until it becomes water and, finally, ice ( Figure 2). In each case, two types of transitions occur, those involving a temperature change with no change in phase (shown by the diagonal line segments on the graphs) and those at constant temperature with a change in phase (shown by horizontal line segments on the graphs). Physical constants The constants for H2O are shown here: Specific heat of ice: Sice = 2.09 J/(g.C) Specific heat of liquid water: Swater = 4.18 J/(g C) Enthalpy of fusion (H2O(s)H2O(1)): AHfus = 334 J/g Enthalpy of vaporization (H2O(1)H2O(g)): Hvap = 2250 J/g Part A How much heat energy, in kilojoules, is required to convert 44.0 g of ice at -18.0C to water at 25.0C? Express your answer to three significant figures and include the appropriate units. View Available Hint(s) Figure Heating curve Temperature (C) 100 Liquid and gas in equilibrium Solid and liquid/ in equilibrium Liquid Vapor Units Value Submit 1 of 2 Part B ? How long would it take for 1.50 mol of water at 100.0 C to be converted completely into steam if heat were added at a constant rate of 24.0 J/s ? Express your answer to three significant figures and include the appropriate units. View Available Hint(s) A ? Units 0 Solid Value Heat added (J) Submit < 7 of 20 Review | Constants | Periodic Table
Step by Step Solution
There are 3 Steps involved in it
Lets break down the problems step by step Part A To convert 440 g of ice at 180 C to water at 2150 C we need to calculate the heat energy required for ... View full answer

Get step-by-step solutions from verified subject matter experts


