Question: Heating solid sodium bicarbonate in a closed vessel establishes the following equilibrium: 2NaHCO3(s)Na2CO3(s)+H2O(g)+CO2(g)H=+129.2kJ Which direction would the equilibrium shift (left to right or right to
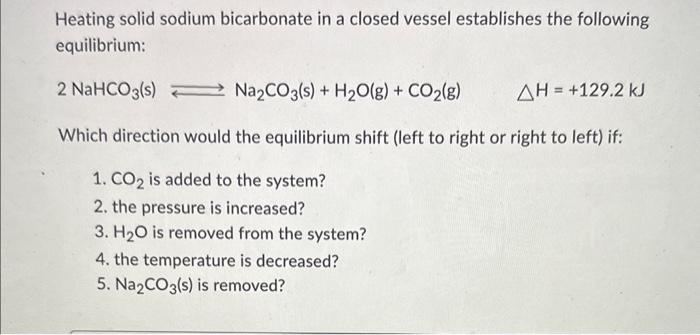
Heating solid sodium bicarbonate in a closed vessel establishes the following equilibrium: 2NaHCO3(s)Na2CO3(s)+H2O(g)+CO2(g)H=+129.2kJ Which direction would the equilibrium shift (left to right or right to left) if: 1. CO2 is added to the system? 2. the pressure is increased? 3. H2O is removed from the system? 4. the temperature is decreased? 5. Na2CO3(s) is removed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


