Question: 3. Calculate the work done, W=mgh for a mass of 300 g. Then, using the accepted value for the specific heat of water, calculate
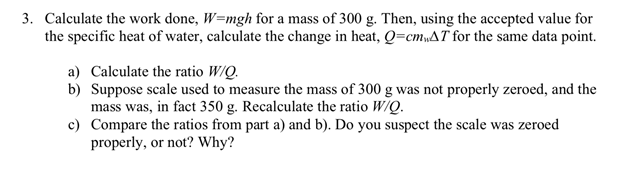
3. Calculate the work done, W=mgh for a mass of 300 g. Then, using the accepted value for the specific heat of water, calculate the change in heat, Q=cmAT for the same data point. a) Calculate the ratio W/Q. b) Suppose scale used to measure the mass of 300 g was not properly zeroed, and the mass was, in fact 350 g. Recalculate the ratio W/Q. c) Compare the ratios from part a) and b). Do you suspect the scale was zeroed properly, or not? Why?
Step by Step Solution
3.39 Rating (143 Votes )
There are 3 Steps involved in it
mgk 1 CAT 981 31x 1499 4018 x 103x 2 1758 X 103 ... View full answer

Get step-by-step solutions from verified subject matter experts


