Question: Hello, can someone please help me get solve these chemistry questions for class ? It is very urgent that Questions up to 31-40 are answered






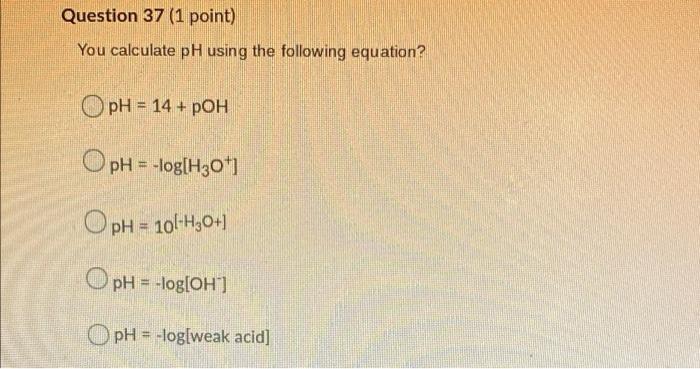

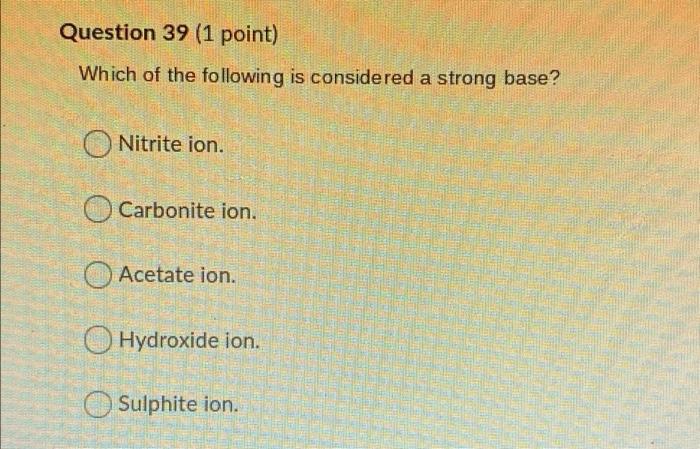

Question 31 (1 point) When a reactant or product is added to a system at equilibrium, the equilibrium will always shift from the substance added. no shift occurs towards away right left Question 32 (1 point) A reaction has a Ko value of 4 8 x 105 What are predominately found at equilibrium? X An equal mixture of reactants and products. Elves. Reactants. You cannot tell from the Kc. Products. Question 33 (1 point) This is calculated and compared to Ko to determine the direction of an equilibrium shift. The reaction quorum. The reaction coordinate. The reaction quotient. The reaction director. The reaction rate. Question 34 (1 point) This simplification for equilibrium problems involves dividing the initial concentration by the Kc. The Perfect Square. The Hundred's rule. The Kc Rule. The Mason Rule. The Thousand's rule. Question 35 (1 point) You calculate the lon Product for Water by subtracting Kb from Ka. dividing Ka by Kb. subtracting Ka from Kb. O multiplying Ka and Kb. O adding Ka and Kb. Question 36 (1 point) The value for Kw (temperature is 25C) is 100 O 10-14 O 107 O 107 O 1014 Question 37 (1 point) You calculate pH using the following equation? OpH = 14 + POH OpH = -log[H301 pH = 10l-H30+) 1 OpH = -log[OH] ) pH = -log[weak acid] Question 38 (1 point) Which of the following acids is considered a strong acid? Sulfurous acid Carbonous acid Nitrous acid Chloric acid Chlorous acid Question 39 (1 point) Which of the following is considered a strong base? Nitrite ion. Carbonite ion. Acetate ion. Hydroxide ion. Sulphite ion. Question 40 (1 point) Which of the following compounds/ions/atoms/molecules do not hydrolyze? O Nal+ OK1+ O Br1- Och- all of the above
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


