Question: Hello, I could use some help to figure this out I dont understand this graph Question III- Consider the given concentration - time diagram (that
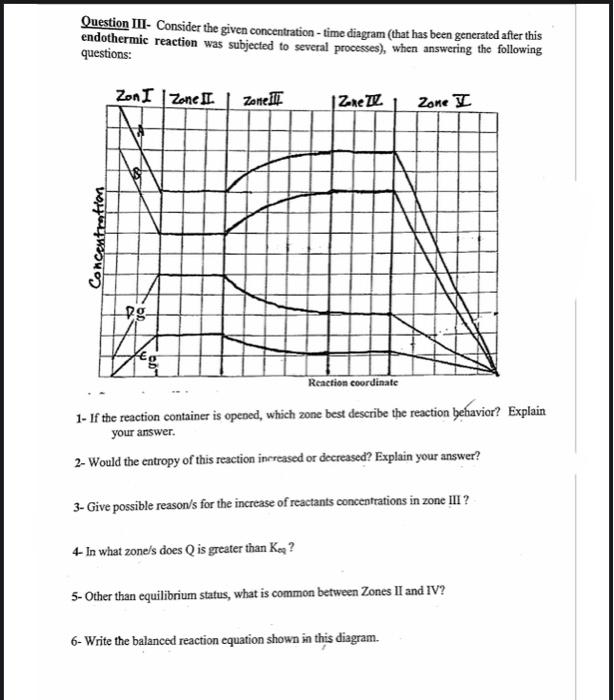
Question III- Consider the given concentration - time diagram (that has been generated after this endothermic reaction was subjected to several processes), when answering the following questions: 1- If the reaction container is opened, which zone best describe the reaction behavior? Explain your answer. 2- Would the entropy of this reaction increased or decreased? Explain your answer? 3- Give possible reason/s for the increase of reactants concentrations in zone III? 4- In what zone/s does Q is greater than Ken ? 5- Other than equilibrium status, what is common between Zones II and IV? 6- Write the balanced reaction equation shown in this diagram. Question III- Consider the given concentration - time diagram (that has been generated after this endothermic reaction was subjected to several processes), when answering the following questions: 1- If the reaction container is opened, which zone best describe the reaction behavior? Explain your answer. 2- Would the entropy of this reaction increased or decreased? Explain your answer? 3- Give possible reason/s for the increase of reactants concentrations in zone III? 4- In what zone/s does Q is greater than Ken ? 5- Other than equilibrium status, what is common between Zones II and IV? 6- Write the balanced reaction equation shown in this diagram
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


