Question: Hello, I have completed everything else. Just need help with question 4 and 5 please Amount of NaOH Prepare a figure (computer generated) of your
 Hello, I have completed everything else. Just need help with question 4 and 5 please
Hello, I have completed everything else. Just need help with question 4 and 5 please
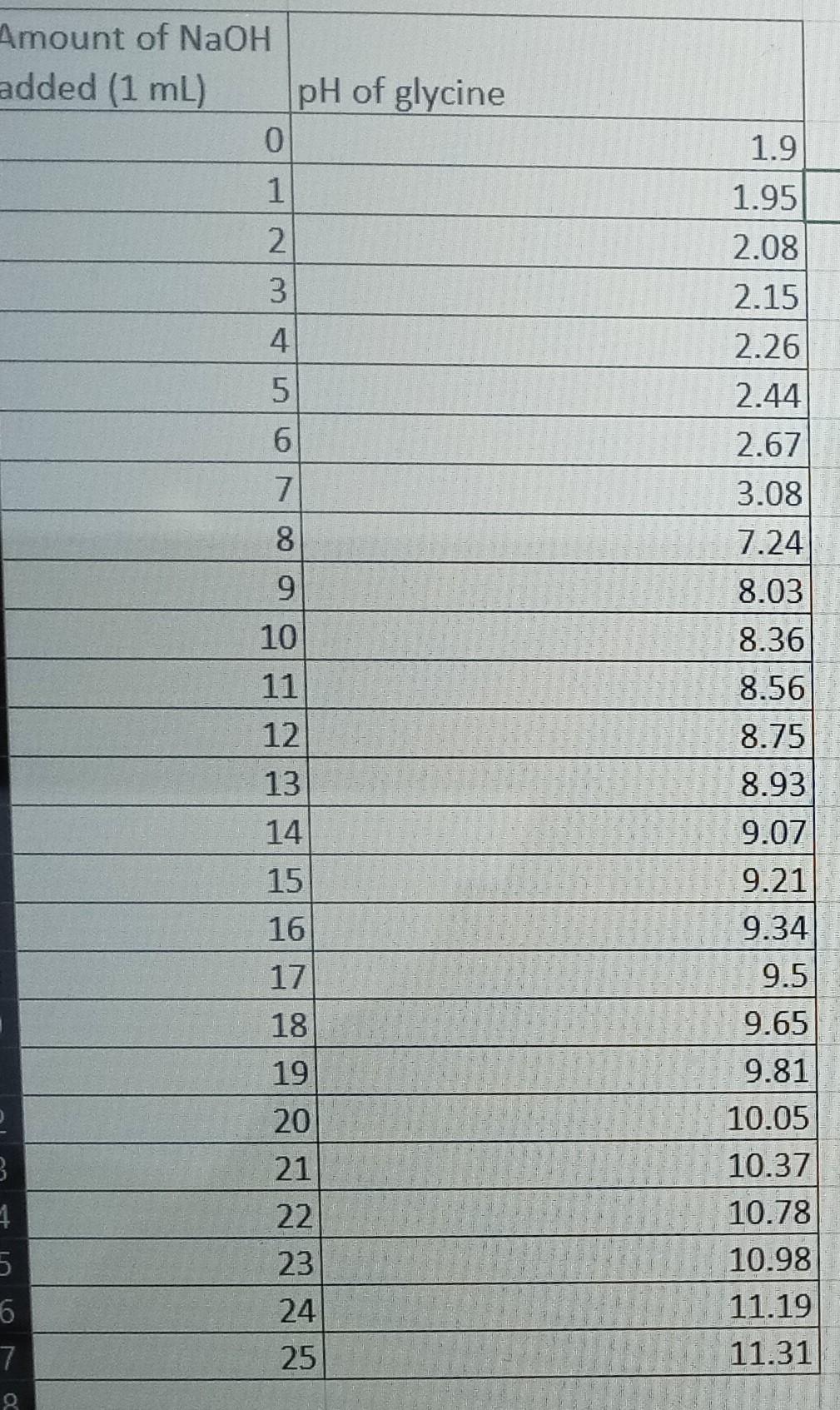
Amount of NaOH Prepare a figure (computer generated) of your glycine titration curve from Part 4. pH should be plotted on the y-axis and ml of NaOH added on the x-axis. Be sure it is appropriately numbered and contains a one-sentence descriptive figure legend ( 8 points): (i) Figure No. and legend (1 point) (ii) axis labels ( 2 points) (iii) titration curve from plotted pH values. Curve can be hand drawn but must connect plotted points ( 1 point) (iv) estimate of pKa1 and pKa2 values (Identify and estimate on graph) (2 points) (v) drawing of correct glycine ionization reactions at appropriate pKa1 and pKa2 points on graph. Circle functional group involved in loss of protons ( 2 points) Amount of NaOH Prepare a figure (computer generated) of your glycine titration curve from Part 4. pH should be plotted on the y-axis and ml of NaOH added on the x-axis. Be sure it is appropriately numbered and contains a one-sentence descriptive figure legend ( 8 points): (i) Figure No. and legend (1 point) (ii) axis labels ( 2 points) (iii) titration curve from plotted pH values. Curve can be hand drawn but must connect plotted points ( 1 point) (iv) estimate of pKa1 and pKa2 values (Identify and estimate on graph) (2 points) (v) drawing of correct glycine ionization reactions at appropriate pKa1 and pKa2 points on graph. Circle functional group involved in loss of protons ( 2 points)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


