Question: Hello, I need help answering the following question. It is a Boiling Heat Transfer Experiment. Measurements: Temp (Celsius) |Pressure (KN/m^2) |Pressure (Bar Gauge) | Pressure
Hello,
I need help answering the following question. It is a Boiling Heat Transfer Experiment.
Measurements:
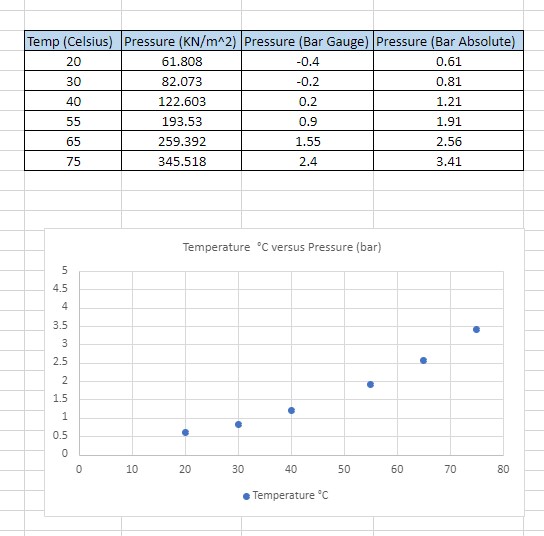
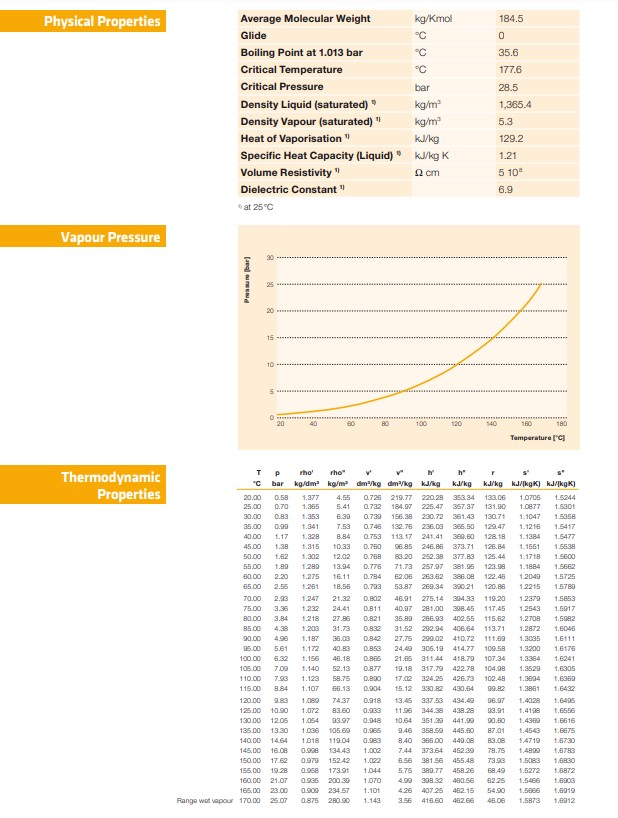




Temp (Celsius) |Pressure (KN/m^2) |Pressure (Bar Gauge) | Pressure (Bar Absolute) 20 61.808 0.4 0.61 30 82.073 -0.2 0.81 40 122.603 0.2 1.21 55 193.53 0.9 1.91 65 259.392 1.55 2.56 75 345.518 2.4 3.41 Temperature "C versus Pressure (bar) 4. 3. in 2.5 1.5 0.5 10 20 30 40 50 60 70 80 . Temperature "CPhysical Properties Average Molecular Weight kg/Kmol 184.5 Glide 0 Boiling Point at 1.013 bar 35.6 Critical Temperature 177.6 Critical Pressure bar 28.5 Density Liquid (saturated) " kg/m 1,365.4 Density Vapour (saturated) " kg/m 5.3 Heat of Vaporisation " KJVkg 129.2 Specific Heat Capacity (Liquid) " kJkg K 1.21 Volume Resistivity " Q cm 5 10 Dielectric Constant " 6.9 at 25 0 Vapour Pressure 100 140 180 20 Temperature ['CI who' rho" Thermodynamic T P bar kg/dm' ko/m" dim Vig dm'Vug kling kJ/kg kd/kg kJ/jkgk] kJ/jkgK] Properties 20.00 0.58 1.377 4.55 0.720 210.77 220.28 353.34 123.06 1 0705 1 6244 357.37 131.50 1 0877 1 5301 25.00 0.70 1.305 5.41 0.732 184.07 225.47 0.730 156.38 230.72 361.43 120.71 1-1047 1 5358 30.00 1.353 6.30 0.746 132.76 206.03 365.50 129.47 1-12 16 1 5417 5.00 0.09 1.341 7153 113. 17 241.41 128. 18 1.1384 1 5477 1.17 1.328 O.753 1-1561 1 5638 15.00 1.38 1.315 56.85 246.88 372.71 126.84 1-1718 50.0 1.82 .302 5500 1.80 1.280 13104 71.73 257.07 381.95 123.08 1_1884 1.204 2.20 1275 16.11 386.08 122.46 120. 86 1 2215 1 578 85 00 2.56 1.351 18.50 0.703 53.87 260.34 300.21 46101 275.14 304.33 115.20 1 2379 1 585 70.00 2.93 1.247 21.32 0.802 0.811 281 100 308.45 117.45 1.2543 1.5017 7500 3.36 232 24.4 80 00 3.84 1.218 27.83 0.821 35.80 286.03 402.55 115.62 1.2708 1.5082 8.38 31.73 0.832 21.52 202.94 406.54 113.71 1.2872 85 00 208 90.00 4.96 36.03 0.842 27.76 200.02 410.72 111.60 1.3035 1.611 1 0500 5.61 1.17. 40.83 0.853 24.40 306.19 414.17 109.58 1.3200 1.6241 6.32 46.18 107.34 1.236 100 00 1.186 0.805 21.65 311.44 418.79 104.08 1.3529 106 00 7.09 .140 52.13 10.18 217.79 422.78 426.73 102.48 1.3804 1 10 00 .123 58.75 17.02 324.25 1.3851 1 15.00 8.84 15.12 230182 430.54 B LEE S'E 434.40 1.4028 1.649 120 00 9.83 1.18 0.018 1.6556 125.00 1090 1.072 83 60 0.983 11.56 244.38 438.28 1.4198 1084 251.30 441.90 1.4380 1.6316 130 0D 12105 1.05 0.048 1036 105 60 0.085 046 358.50 445.60 87101 1.4543 135 00 13.30 140.00 1464 1018 11904 0.983 8.40 365.00 440.08 83.08 1.4719 1.678 145.00 0.908 134.43 1.002 7.44 373.64 452.30 78.75 1.4820 150.00 17 62 0.579 152.42 .022 656 381.56 455.48 1.5083 1.683 1.6872 156.00 19 28 0958 173.91 1.044 5.76 380.77 458.26 68.49 1.5272 160.00 21 07 0 935 200 30 1070 208 32 450 58 82.2 1.5486 0900 234 57 1.101 4.26 407-26 462 15 54.90 1.5068 1.6919 165.00 2300 1.142 3.56 462 66 48 08 25 07 0.875 280 90 416.60 1.5873 Range wat vapour 170.00\fComment on the variation of pressure with temperature and comment on whether this is the expected result.c Find published data for saturation temperature versus saturation pressure graph or Ph chart for the solvent used in the experiment and compare with the experimental values (SE36) 0 Were the results as expected and with suitable justification, in your opinion what factors may have contributed to any differences? Draw conclusions from results. The conclusion should summarise what was learned in the laboratory and its relevance to an engineer. [If possible/applicable, give reasons for poor results. Comment on any deficiencies in the equipment or the procedure. If possible, suggest improvements in the equipment or the procedure]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


