Question: Hello, I need help filling out Table 8.3 and Table 8.4, Also it will be much appreciated if Graphs are created too! I need help
Hello, I need help filling out Table 8.3 and Table 8.4, Also it will be much appreciated if Graphs are created too! I need help on those as well!
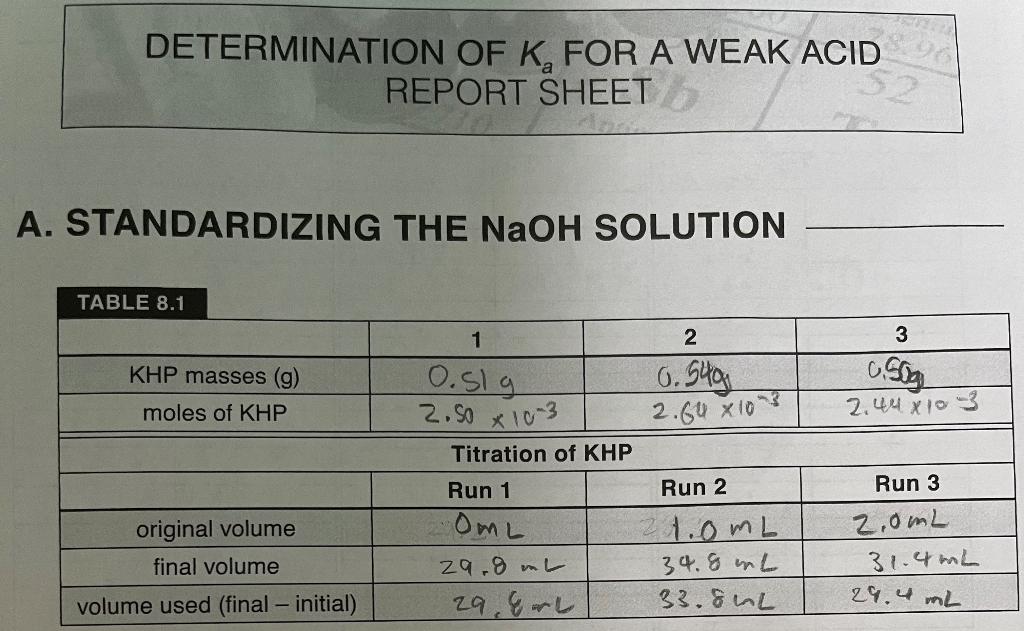
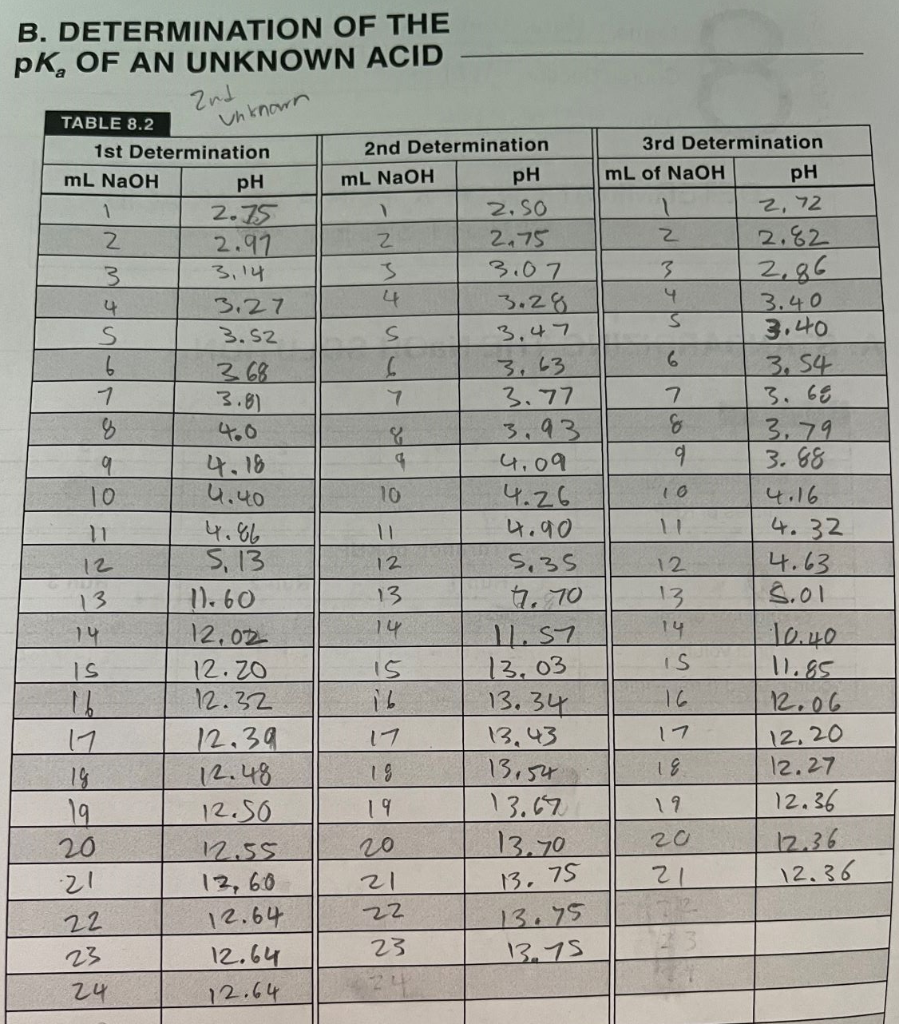
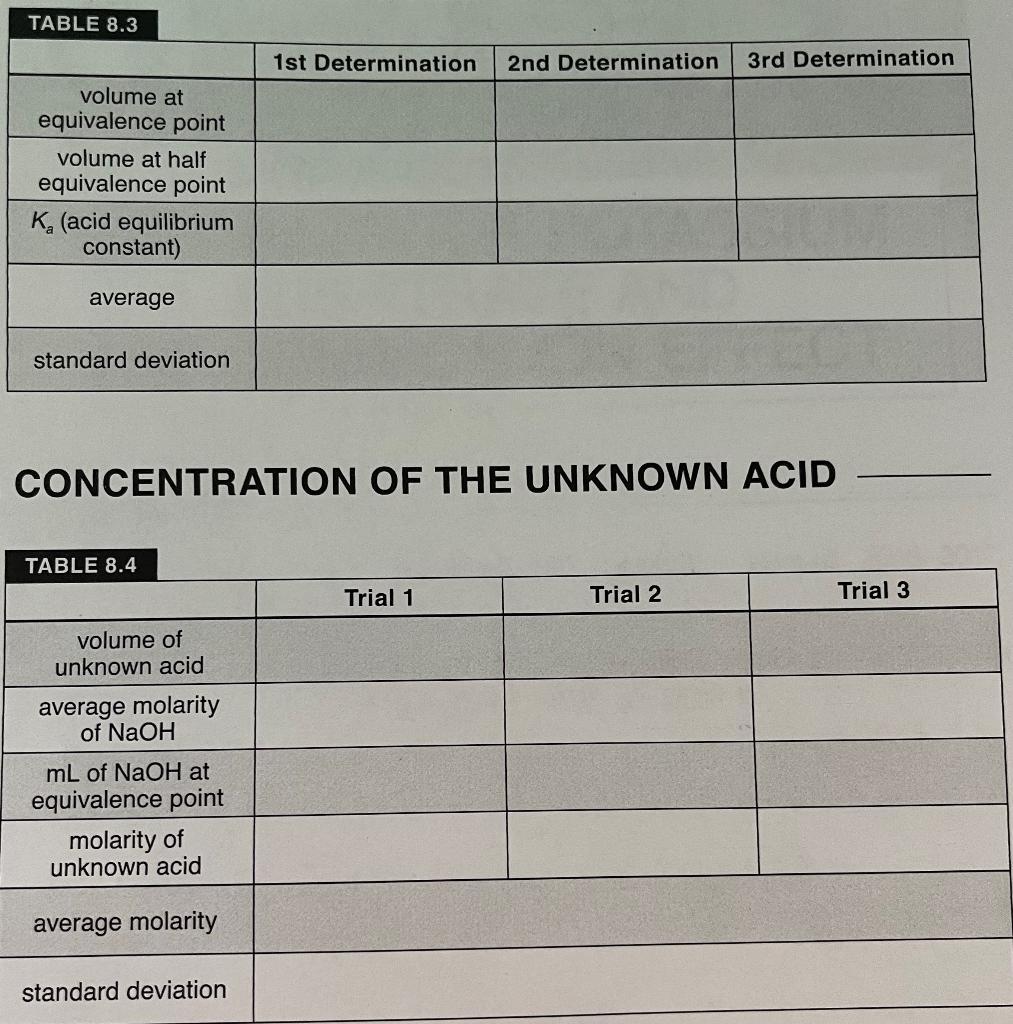
DETERMINATION OF K, FOR A WEAK ACID REPORT SHEET A. STANDARDIZING THE NaOH SOLUTION TABLE 8.1 3 KHP masses (9) 0.51 g c. Sog 2.448103 moles of KHP 2.60 x 10? 1 2 0.54g 2.50 x 103 Titration of KHP Run 1 Run 2 OML 21.0mL 29.8mL 34.8 L 29. &L 33.80L Run 3 original volume final volume volume used (final - initial) 2.0mL 31.4mL 29.4 mL B. DETERMINATION OF THE pK, OF AN UNKNOWN ACID 2nd Unknown 4.18 . TABLE 8.2 1st Determination mL NaOH pH 1 2.75 2 2.97 3. 3,14 . 3.27 S 3.52 6 368 1 3.8) 8 4.0 9 10 11 4.86 12 5.13 13 11.60 14 12,02 IS 12.20 16 12.32 17 12.39 14 2.48 19 12.50 20 12.55 21 13,60 12.64 12.64 24 12.64 2nd Determination ml NaOH pH 2. SO 2 2,75 3 3.07 4 3.28 S 3.47 6 3.63 7 3.77 3.93 4.09 10 4.26 4.90 12 5.35 13. 4. 70 1.57 13.03 16 17 13.43 18 13.54 19 13.67 20 13.70 21 22 13.75 23 13.75 24 3rd Determination mL of NaOH pH 2, 72 z 2.82 3 2.86 4 3.40 3.40 6 3.54 7 3. 68 % 3.79 9 3.68 10 4.16 4.32 12 4.63 13 5.01 14 10.40 IS 11.85 16 12.06 12.0 18 12.27 19 12.36 20 12.36 21 12.36 14 15 13.34 17 13. 75 22 23 TABLE 8.3 1st Determination 2nd Determination 3rd Determination volume at equivalence point volume at half equivalence point K. (acid equilibrium constant) average standard deviation CONCENTRATION OF THE UNKNOWN ACID TABLE 8.4 Trial 1 Trial 2 Trial 3 volume of unknown acid average molarity of NaOH mL of NaOH at equivalence point molarity of unknown acid average molarity standard deviation
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


