Question: Hello, I'm looking for help on these Dimensional Analysis questions. 4. If PV=[gR(T+273.15)]/M, solve for M when P=425,V=0.301,g=0.585,R=62.37, and T=22 5. An ore contains 39.2%
Hello, I'm looking for help on these Dimensional Analysis questions. 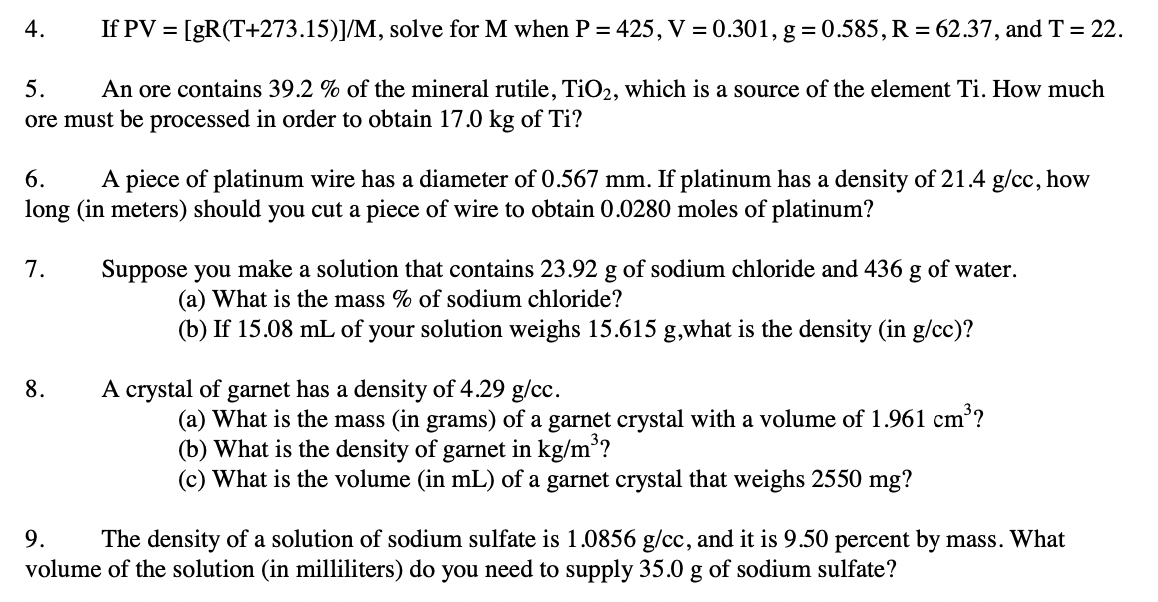
4. If PV=[gR(T+273.15)]/M, solve for M when P=425,V=0.301,g=0.585,R=62.37, and T=22 5. An ore contains 39.2% of the mineral rutile, TiO2, which is a source of the element Ti. How much ore must be processed in order to obtain 17.0kg of Ti? 6. A piece of platinum wire has a diameter of 0.567mm. If platinum has a density of 21.4g/cc, how long (in meters) should you cut a piece of wire to obtain 0.0280 moles of platinum? 7. Suppose you make a solution that contains 23.92g of sodium chloride and 436g of water. (a) What is the mass % of sodium chloride? (b) If 15.08mL of your solution weighs 15.615g,what is the density (in g/cc )? 8. A crystal of garnet has a density of 4.29g/cc. (a) What is the mass (in grams) of a garnet crystal with a volume of 1.961cm3 ? (b) What is the density of garnet in kg/m3 ? (c) What is the volume (in mL ) of a garnet crystal that weighs 2550mg ? 9. The density of a solution of sodium sulfate is 1.0856g/cc, and it is 9.50 percent by mass. What volume of the solution (in milliliters) do you need to supply 35.0g of sodium sulfate
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


