Question: Hello, Please answer the following questions and make answer clear to understand. Review Constants Periodic Table Part A MISSED THIS? Read Section 19.10 (Pages 881
Hello, Please answer the following questions and make answer clear to understand.
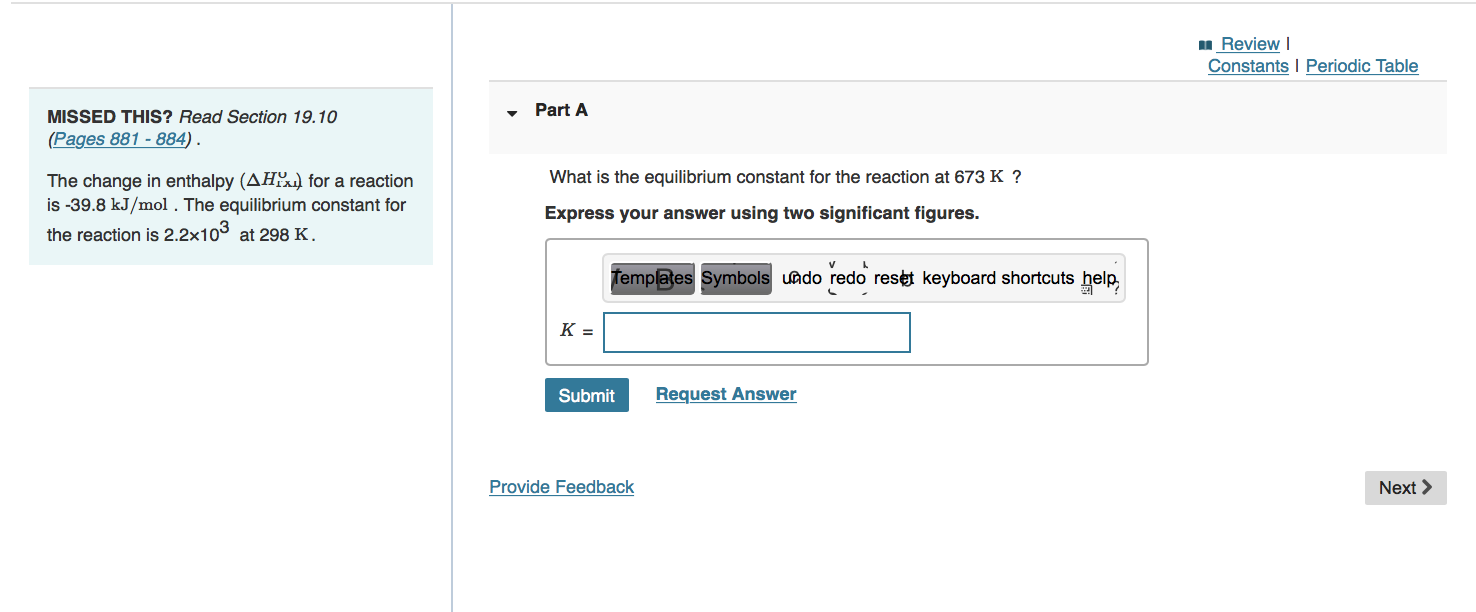
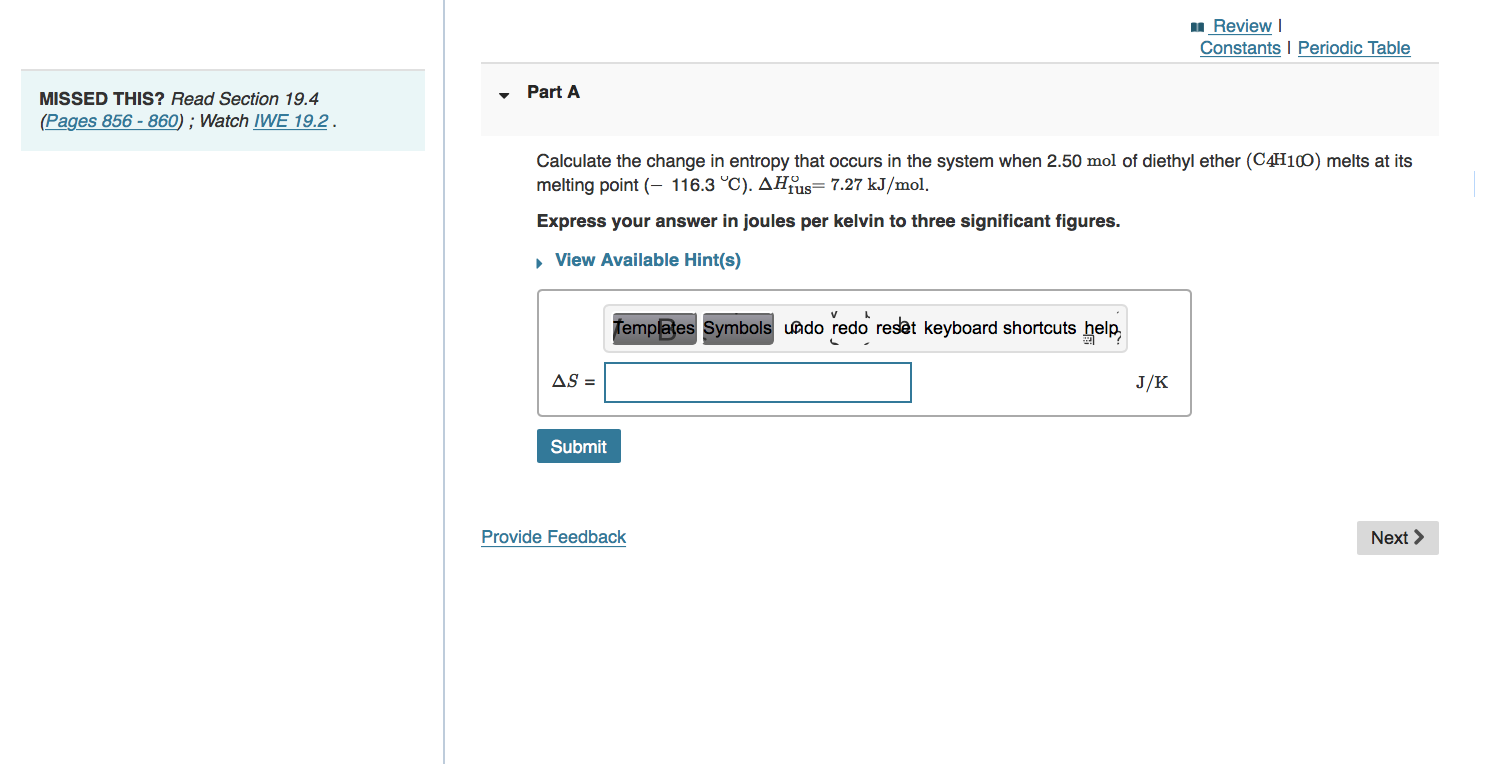
Review Constants Periodic Table Part A MISSED THIS? Read Section 19.10 (Pages 881 - 884). What is the equilibrium constant for the reaction at 673 K ? The change in enthalpy (AHA) for a reaction is -39.8 kJ/mol. The equilibrium constant for the reaction is 2.2x103 at 298 K. Express your answer using two significant figures. templates Symbols Lado redo reset keyboard shortcuts hele K = Submit Request Answer Provide Feedback Next > Review Constants Periodic Table Part A MISSED THIS? Read Section 19.4 (Pages 856 - 860); Watch IWE 19.2. Calculate the change in entropy that occurs in the system when 2.50 mol of diethyl ether (C4H100) melts at its melting point (- 116.3 C). AHjus= 7.27 kJ/mol. Express your answer in joules per kelvin to three significant figures. View Available Hint(s) templates Symbols undo redo rest keyboard shortcuts help AS = J/K Submit Provide Feedback Next >
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


