Question: Hello Please help me i will rate you nicely im not sure how to do this and can use any help. dont have much time.
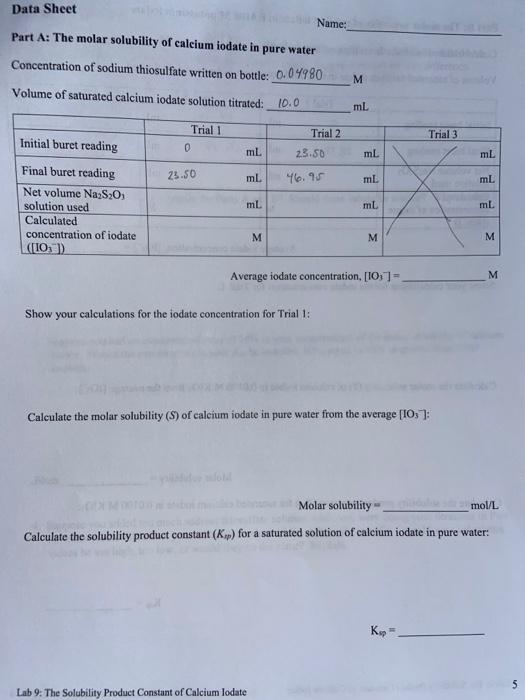
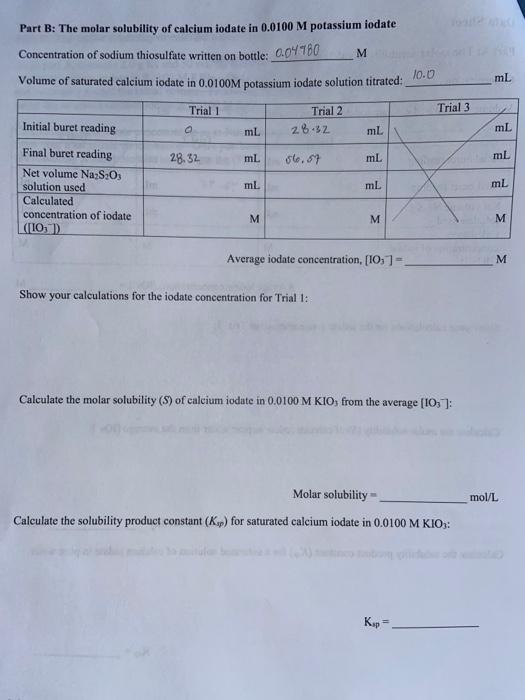

Part A: The molar solubility of calcium iodate in pure water Concentration of sodium thiosulfate written on bottle: 0.04980M Volume of saturated calcium iodate solution titrated: 10.0mL Average iodate concentration, [OO3]= M Show your calculations for the iodate concentration for Trial 1: Calculate the molar solubility (S) of calcium iodate in pure water from the average [OO3] : Molar solubility = mol/L Calculate the solubility product constant (Kmp) for a saturated solution of calcium iodate in pure water: Ksp= Lab 9: The Solubility Product Constant of Calcium lodate Part B: The molar solubility of calcium iodate in 0.0100M potassium iodate Concentration of sodium thiosulfate written on bottle: 0.04980M Volume of saturated calcium iodate in 0.0100M potassium iodate solution titrated: 10.0 Average iodate concentration, [IO3]= M Show your calculations for the iodate concentration for Trial 1: Calculate the molar solubility (S) of calcium iodate in 0.0100MKIO, from the average [IO3] : Molar solubility = mol/L Calculate the solubility product constant (Ksp) for saturated calcium iodate in 0.0100MKIO3 : 1. How did the molar solubility of calcium iodate in pure water compare to its solubility in 0.0100M potassium iodate? If there was a difference in your values, suggest a reason for this difference. 2. How did your Ksp values compare for Parts A and B ? Should these values be similar? Why or why not? 3. Suppose you had an experimental error of 10% in your measurement of the molar solubility of calcium iodate. Would the percent error in your Kp be less than 10%, equal to 10% or greater than 10% ? Explain. 4. Suppose you added too much sodium thiosulfate solution while you were doing your titrations in Part A. How would this affect your calculated values of S and Kpp ? Be specific-would your calculated values be too high, too low, or would they be the same? Explain. Part A: The molar solubility of calcium iodate in pure water Concentration of sodium thiosulfate written on bottle: 0.04980M Volume of saturated calcium iodate solution titrated: 10.0mL Average iodate concentration, [OO3]= M Show your calculations for the iodate concentration for Trial 1: Calculate the molar solubility (S) of calcium iodate in pure water from the average [OO3] : Molar solubility = mol/L Calculate the solubility product constant (Kmp) for a saturated solution of calcium iodate in pure water: Ksp= Lab 9: The Solubility Product Constant of Calcium lodate Part B: The molar solubility of calcium iodate in 0.0100M potassium iodate Concentration of sodium thiosulfate written on bottle: 0.04980M Volume of saturated calcium iodate in 0.0100M potassium iodate solution titrated: 10.0 Average iodate concentration, [IO3]= M Show your calculations for the iodate concentration for Trial 1: Calculate the molar solubility (S) of calcium iodate in 0.0100MKIO, from the average [IO3] : Molar solubility = mol/L Calculate the solubility product constant (Ksp) for saturated calcium iodate in 0.0100MKIO3 : 1. How did the molar solubility of calcium iodate in pure water compare to its solubility in 0.0100M potassium iodate? If there was a difference in your values, suggest a reason for this difference. 2. How did your Ksp values compare for Parts A and B ? Should these values be similar? Why or why not? 3. Suppose you had an experimental error of 10% in your measurement of the molar solubility of calcium iodate. Would the percent error in your Kp be less than 10%, equal to 10% or greater than 10% ? Explain. 4. Suppose you added too much sodium thiosulfate solution while you were doing your titrations in Part A. How would this affect your calculated values of S and Kpp ? Be specific-would your calculated values be too high, too low, or would they be the same? Explain
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


