Question: hello, please help me solve this one the changes in pH that occur. Prepare buffer solutions. 1. At one of the buret stations, fill the
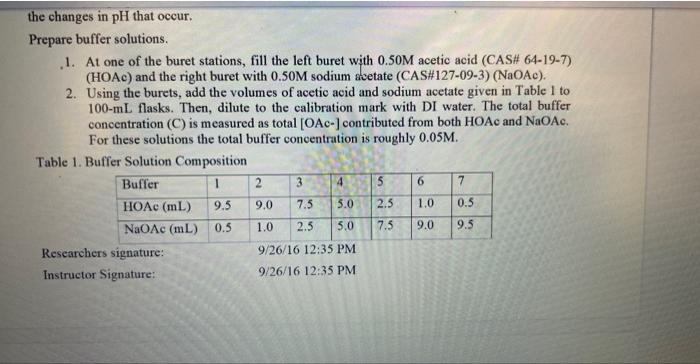
the changes in pH that occur. Prepare buffer solutions. 1. At one of the buret stations, fill the left buret with 0.50M acetic acid (CAS\# 64-19-7) (HOAc) and the right buret with 0.50M sodium avetate (CAS\#127-09-3) (NaOAc). 2. Using the burets, add the volumes of acetic acid and sodium acetate given in Table 1 to 100-mL flasks. Then, dilute to the calibration mark with DI water. The total buffer concentration (C) is measured as total [OAc-] contributed from both HOAc and NaOAc. For these solutions the total buffer concentration is roughly 0.05M. Table 1. Buffer Solution Composition Q2 Prediction/Hypothesis 10 Points 6. Hypothesis: In Table 1, Which solution(s) do you think will have the highest buffer capacity? Why? Enter your answer here
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


