Question: Hello, please help with this question. I have no idea how to solve this so it would help a lot if you could explain it
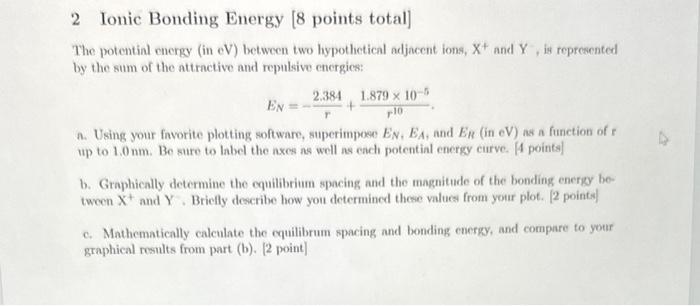
2 Ionic Bonding Energy [8 points total] The potentinl energy (in eV) between two hypothetical adjacent ions, X+and Y, is represented by the sum of the attractive and repulsive energics: EN=r2.384+r101.879105. a. Using your favorite plotting software, superimpoee EN,EA, and ER (in eV ) as a function of r up to 1.0nm. Be sure to label the nxes as well as each potential energy curve. [4 points] b. Graphically determine the equilibrium spacing and the magnitude of the bonding energy between X+and Y. Briefly describe how you determined these values from your plot. [2 pointes] c. Mathematically calculate the equilibrum spacing and bonding energy, and compare to your graphical results from part (b). [2 point]
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


