Question: help 30 Lithium and nitrogen react in a combination reaction to produce lithlum nitride: 6Li(s)+N2(g)2Li3N(s) In a particular experiment, 2.50g samples of each reagent (
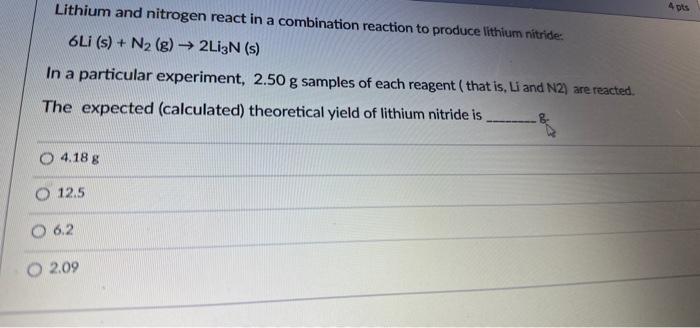
Lithium and nitrogen react in a combination reaction to produce lithlum nitride: 6Li(s)+N2(g)2Li3N(s) In a particular experiment, 2.50g samples of each reagent ( that is, L and N2 ) are reacted. The expected (calculated) theoretical yield of lithium nitride is 4.18g 12.5 6.2 2.09
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


