Question: Help answer question 9 9. Write each of the 3 reactions in your notebook. Follow the instructions below for how to enter the reactions. Write
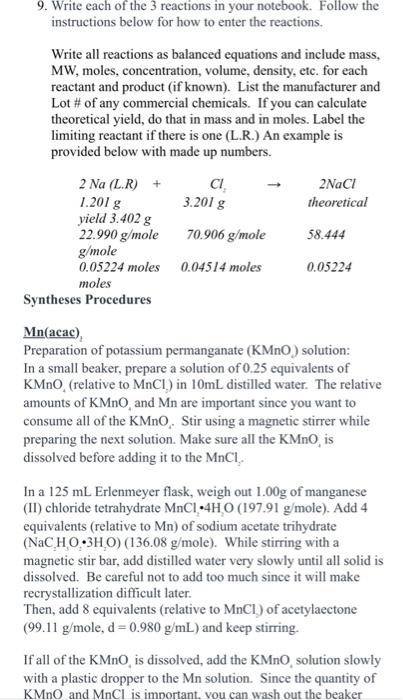


9. Write each of the 3 reactions in your notebook. Follow the instructions below for how to enter the reactions. Write all reactions as balanced equations and include mass, MW, moles, concentration, volume, density, etc. for each reactant and product (if known). List the manufacturer and Lot \# of any commercial chemicals. If you can calculate theoretical yield, do that in mass and in moles. Label the limiting reactant if there is one (L.R.) An example is provided below with made up numbers. Synthi Mn(acac) Preparation of potassium permanganate (KMnO) solution: In a small beaker, prepare a solution of 0.25 equivalents of KMnO4 (relative to MnCl2 ) in 10mL distilled water. The relative amounts of KMnO4 and Mn are important since you want to consume all of the KMnO*. Stir using a magnetic stirrer while preparing the next solution. Make sure all the KMnO, is dissolved before adding it to the MnCl.. In a 125mL Erlenmeyer flask, weigh out 1.00g of manganese (II) chloride tetrahydrate MnCl24HO ( 197.91g/ mole). Add 4 equivalents (relative to Mn ) of sodium acetate trihydrate (NaC2H3O23H2O)(136.08g/ mole ). While stirring with a magnetic stir bar, add distilled water very slowly until all solid is dissolved. Be careful not to add too much since it will make recrystallization difficult later. Then, add 8 equivalents (relative to MnCl ) of acetylaectone (99.11g/mole,d=0.980g/mL) and keep stirring. If all of the KMnO4 is dissolved, add the KMnO4, solution slowly with a plastic dropper to the Mn solution. Since the quantity of KMnO and MnCl is imnortant. vou can wash out the beaker Fe(acac)2 Dissolve 3 mmoles of iron(III) nitrate nonahydrate Fe(NO)3H2O(404g/mole) in 6mL of distilled water in a small Erlenmeyer flask with magnetic stirring. Dissolve 3.1 equivalents (relative to Fe ) of acetylacetone (99.11 g/mole,d=0.980g/mL ) in 6mL of methanol and add that to the reaction mixture. Dissolve 3.1 equivalents (relative to Fe ) sodium acetate trihydrate (NaC2H3O23H2O)(136.08g/ mole ) in 6mL of distilled water. Add to the reaction mixture, stir briefly, and then heat on a sand bath to boil off the methanol. Use the method you described in your prelab to determine when the volume has decreased by 68mL. Place the flask on the bench to cool and then place it in an ice bath for 10 minutes or so, until crystals form. Filter the solid using a Hirsh or Buchner funnel. Rinse 2 times with 5mL of distilled water. After the initial filtration, put each rinse in the original reaction flask, scrape off any chunks and then quickly transfer to the filter. Place your product in a tared vial (measure the mass of the empty vial with the lid so that you can determine the mass of the contents on another day). Determine the mass of the wet product. Let your product dry in the inorganic drawer for the next week with the lid off of the vial. Determine the mass of the dry product. Cr(acac) Heat a water bath (beaker of water large enough to fit your reaction flask) on a sandbath. Weigh 1.30g of CrCl36H2O into a 50 -mL Erlenmeyer flask. Add 20.0mL of distilled water, and stir briefly. When the chromium compound has dissolved, add 5.0g of urea and 4.0 mL of acetylacetone. Using a ring-stand and clamp, place the reaction vessel in a water bath on your sand bath, ensuring that the flask does not touch the bottom of the water bath and that the liquid level in your flask remains below the level of water in your water bath. Refill the water bath as necessary. Stir the reaction using a glass stir rod and heat the reaction to a temperature greater than 90C. Heat the mixture, uncovered and with vigorous stirring every few minutes, for approximately one hour. As the urea releases ammonia and the solution becomes basic, deep maroon crystals will begin to form. After one hour, allow the reaction to cool to room temperature, and then place it in an ice bath to complete crystallization (Note: if you do not see solid after 1h, continue to heat and stir until you do; sometimes it takes a bit longer!). Collect the product by vacuum filtration using a Bchner funnel. Wash out the flask and the crystals on the filter with plenty of deionized water (about 3 rinses of 10mL each). After the initial filtration, put each rinse in the original reaction flask, quickly scrape off any chunks and then quickly transfer to the filter. Place your product in a tared vial (measure the mass of the empty vial with the lid so that you can determine the mass of the contents on another day). Determine the mass of the wet product. Let your product dry in the inorganic drawer for the next week with the lid off of the vial. Determine the mass of the dry product
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


