Question: help Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest
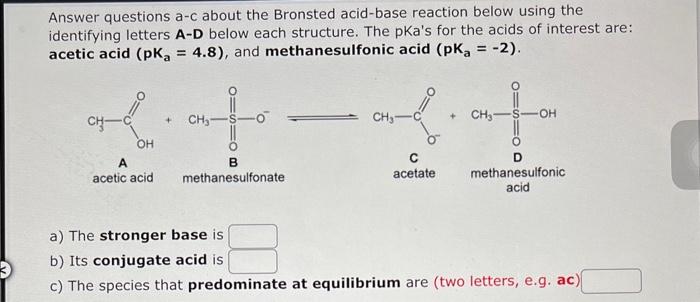
Answer questions a-c about the Bronsted acid-base reaction below using the identifying letters A-D below each structure. The pKa's for the acids of interest are: acetic acid (pKa=4.8), and methanesulfonic acid (pKa=2). a) The stronger base is b) Its conjugate acid is c) The species that predominate at equilibrium are (two letters, e.g. ac)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


