Question: HELP ASAP Consider methylenecyclopropene whose structure is shown below. 1 2 3 4 a) Write down the Hckel Hamiltonian for this system. Use Hi =
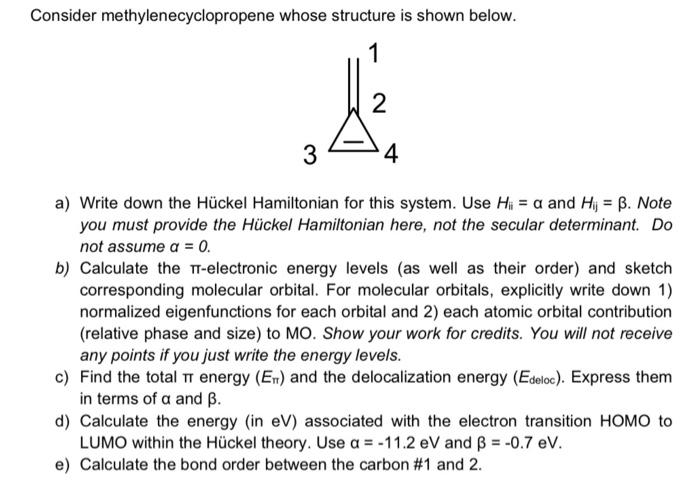
Consider methylenecyclopropene whose structure is shown below. 1 2 3 4 a) Write down the Hckel Hamiltonian for this system. Use Hi = a and Hi = B. Note you must provide the Hckel Hamiltonian here, not the secular determinant. Do not assume a = 0. b) Calculate the T-electronic energy levels (as well as their order) and sketch corresponding molecular orbital. For molecular orbitals, explicitly write down 1) normalized eigenfunctions for each orbital and 2) each atomic orbital contribution (relative phase and size) to MO. Show your work for credits. You will not receive any points if you just write the energy levels. c) Find the total tr energy (En) and the delocalization energy (Edeloc). Express them in terms of a and B. d) Calculate the energy (in eV) associated with the electron transition HOMO to LUMO within the Hckel theory. Use a = -11.2 eV and = -0.7 eV. e) Calculate the bond order between the carbon #1 and 2
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


