Question: help asap....question 1 i need help with questine 1 & 2 and the graphs please Part B 1. Calculate the units of enzyme activity for
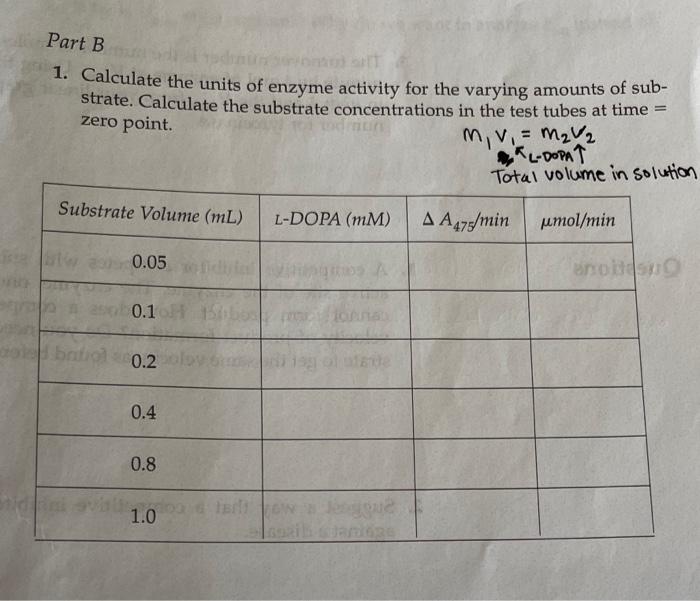
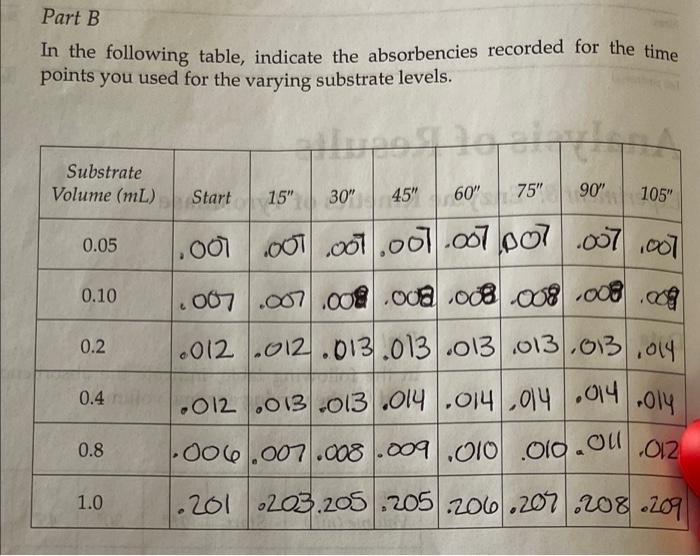

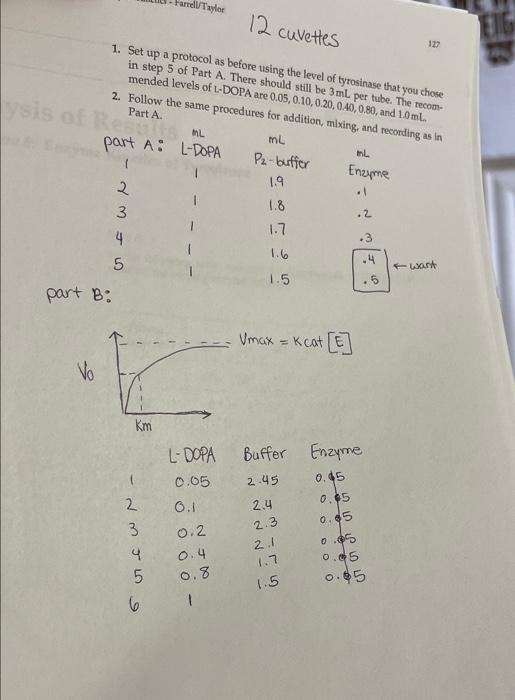
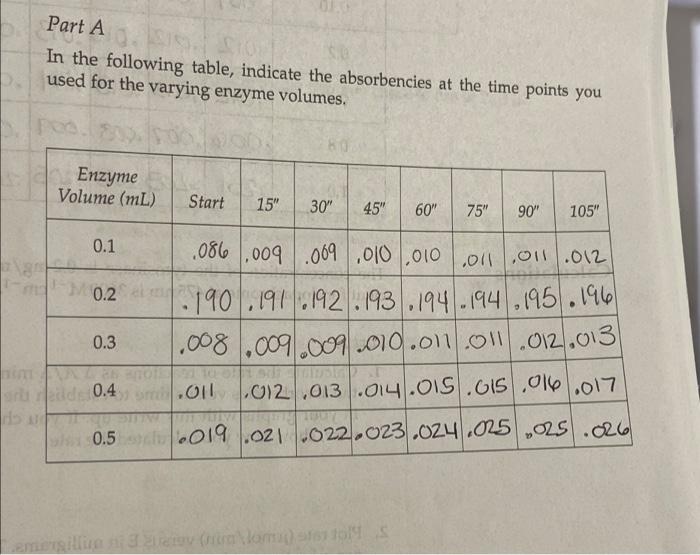
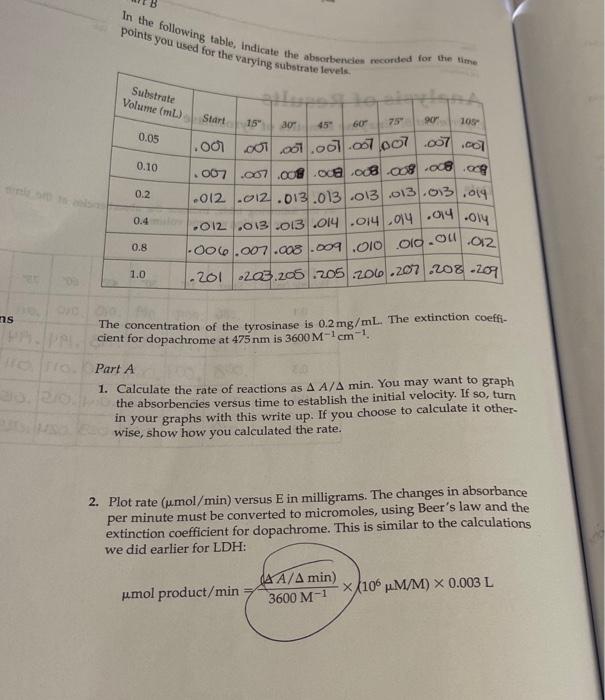
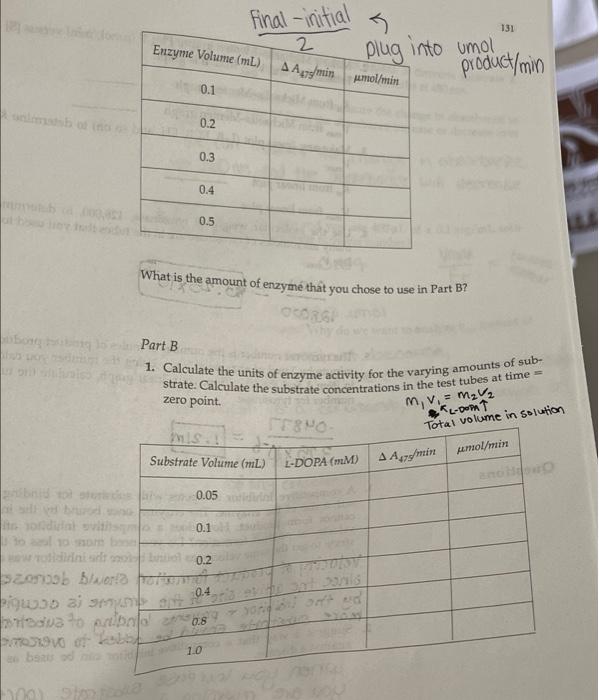
Part B 1. Calculate the units of enzyme activity for the varying amounts of sub- strate. Calculate the substrate concentrations in the test tubes at time zero point. m, v. = MV2 **L-DOPAT Total volume in solution Substrate Volume (mL) L-DOPA (MM) A A475/min pmol/min 0.05 aro 0.1 brito 0.2 0.4 0.8 dir ent 1.0 Part B In the following table, indicate the absorbencies recorded for the time points you used for the varying substrate levels. Substrate Volume (mL) Start 15" 30" 45" 60" 75" 90" 105" 0.05 0.10 0.2 1.000.000 .001.007.007 007 007 1007 2007 .007 .000 .000 .008008 .008.09 1.012.012.013.013/.013 013.03.014 1.012.013.013.014.014 014.014 006.007.008.009.010 .010.00 0.4 Oly 0.8 .012 1.0 .201 203 205.205.206.207 208.209 Reaction: Enamenalo plor Tyrosine L-DOPA Dopachrome (yellow) (no color) Design protocols for determining kinetic parameters Explain the significance of Vanda V Materials Sodium phosphate buffer, 0.1 M pH 7.0 Mushroom tyrosinase (0.2 mg/mL) L-DOPA, 15 mm Spectrophotometers Cuvettes Dom ental res Methods Part A: Determining a Tyrosinase Level for Kinetic Assays In this part of the experiment, you will determine the proper amount of tyrosinase to use for the kinetic analyses. If too little enzyme is used the the change in Aes will be too small to detect, especially at low substrate On the other hand, if too much is used the substrate will be depleted to quickly, and the rate will not be linear for a measurable time. For the dopachrome assay you want a linear rate for at least 1 min while taking lesstime 1. Set up a protocol for the determination of the correct tyrosinase con centration. Each tube should have a total of 3mL of solution and be 5 mM in L-DOPA. The volume will be controlled by the phosphate buffer, and the amount of enzyme will vary. Do at least five tubes ranging from 0.1 to 0.5 mL of tyrosinase. 2. Pipet the L-DOPA and phosphate buffers. 3. Pipet the chosen amount of enzyme into one tube. Mix by inversion and immediately read the change in absorbance at 475 nm. 4. Measure for 2 min, recording at 15-s intervals. Repeat steps 24 for the other enzyme concentrations. Remember: Add the enzyme immediately before reading each tube! 5. Determine the rate for five different tyrosinase levels. The final amount chosen for the next part should give you a change in absorbance per minute (AA/A min) between 0.2 and 03/min. The levels given in step 1 are only suggestions. You may have to try higher or lower volumes. Part B: Determining Kinetic Constants of Tyrosinase Now that the appropriate enzyme level has been determined, the kinetic parameters, KV will be determined. In Part A, the L-DOPA was saturating. In this part, nonsaturating levels will be used. and kort Farrell Taylor 12 cuvettes 122 1. Set up a protocol as before using the level of tyrosinase that you chose in step 5 of Part A. There should still be 3 ml per tube. The recom- mended levels of L-DOPA are 0.05,0.10,0.20,040,0.80, and 10 ml. 2. Follow the same procedures for addition, mixing, and recording as in Part A lysis of ML mL part A: L-DOPA IL ( P2-buffer Enzyme . 3 1.9 1.8 1.7 .2 of we .3 1.6 .4 4 5 part B: I want 1.5 5 Umax = K cat ] Vo Km Buffer Enzyme L-DOPA 0.05 2.45 2.4 0.1 2 3 2.3 6 voc wr- 0.45 0.05 0.95 0.95 0.05 0.45 0.2 0.4 0.8 2.1 5 1.7 1.5 1 Part A In the following table, indicate the absorbencies at the time points you used for the varying enzyme volumes, Bio Enzyme Volume (mL) Start 15" 30" 45" 60" 75" 90" 105" . 0.1 .086.009 .009 010 0100111.011/.012 10.2. 190 191 192 193 194 194 195 196 0.3 1.008.009.009 010.01.011.012.013 0.4 .011 1.012.013.014.015.05.016.017 0019.021.022.023.024.025 .025.026 SE 0.5 millia (nom) 11619 In the following table, indicate the absorbendes recorded for the time points you used for the varying substrate levels Substrate Volume (mL) Start 15" 60 90 105 0.05 0.10 0.2 ac .001 01.01.001.067.007 .007.00 .007 .007 008 .oce 08.03 .008 og .012 2012.013.013 .013 .013.013.019 1.012 .013 013 .014.014014014.oly 1.006.007.008.009 .010 010.on 012 -201 .203.205.205.200.207.208.209 0.4 0.8 1.0 ms The concentration of the tyrosinase is 0.2 mg/mL. The extinction coeffi- cient for dopachrome at 475 nm is 3600M-1 cm Part A 1. Calculate the rate of reactions as A A/A min. You may want to graph the absorbencies versus time to establish the initial velocity. If so, turn in your graphs with this write up. If you choose to calculate it other- wise, show how you calculated the rate. 2. Plot rate (umol/min) versus E in milligrams. The changes in absorbance per minute must be converted to micromoles, using Beer's law and the extinction coefficient for dopachrome. This is similar to the calculations we did earlier for LDH: umol product/min (4A/A min) 3600 M-1 x 106 M/M) X 0.003 L Final -initial 131 Enzyme Volume (mL) 2 A Amin umol/min plug into umol product/min 0.1 0.2 0.3 0.4 0.5 What is the amount of enzyme that you chose to use in Part B? GEORG Part B 1. Calculate the units of enzyme activity for the varying amounts of sub- strate. Calculate the substrate concentrations in the test tubes at time zero point. rrayo M, MgV2 L-Dom 1 Total volume in Solution IS umol/min Substrate Volume (ML) L-DOPA (MM) A 447 min no bado 0.05 0.1 0.2 to rondital svit to onda szorob blword from Wo ai suo 0.4 da to bola 0.90 TL 08 1.0 SO bos
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


