Question: help clarify, i got the answer -58 kJ/mol. is this correct? While HBr in the presence of peroxide initiator efficiently hydrohalogenates alkenes in Anti- Markovnikov
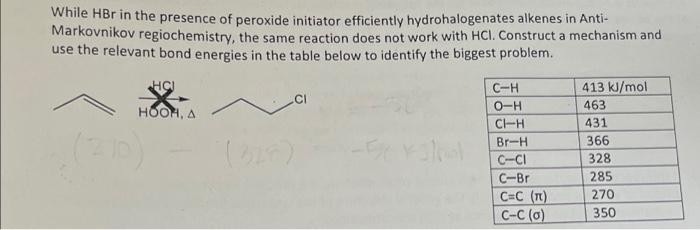
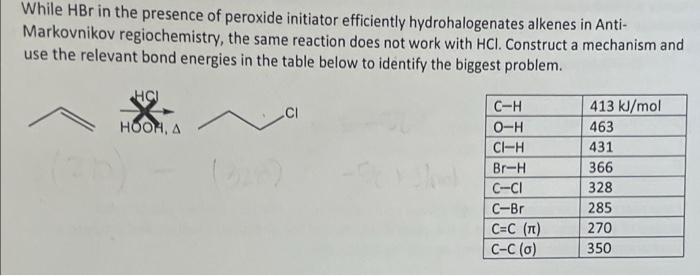
While HBr in the presence of peroxide initiator efficiently hydrohalogenates alkenes in Anti- Markovnikov regiochemistry, the same reaction does not work with HCl. Construct a mechanism and use the relevant bond energies in the table below to identify the biggest problem. C-H 413 kJ/mol HOOMA O-H 463 CI-H 431 Br-H 366 C--CI 328 C-Br 285 C=C (T) 270 C-C(0) 350 While HBr in the presence of peroxide initiator efficiently hydrohalogenates alkenes in Anti- Markovnikov regiochemistry, the same reaction does not work with HCl. Construct a mechanism and use the relevant bond energies in the table below to identify the biggest problem. HCU CI HOOMA C-H O-H CI-H Br-H C-CI C-Br C=C (TL) C-C(O) 413 kJ/mol 463 431 366 328 285 270 350
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


