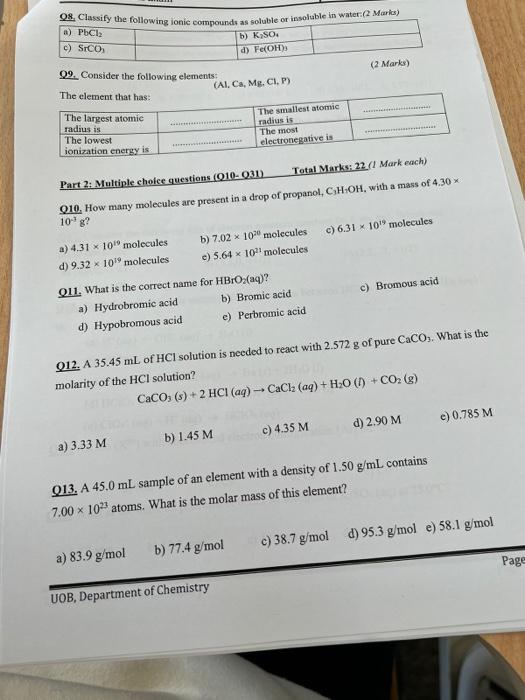
Question: help!!! fast!!! Q9. Consider the following elements: (Al, Ca, Mg, Cl, P) Part 2: Multiple choice questions (Q10- Q31) Total Marks: 22 (1 Mark each)
 fast!!!
fast!!!Q9. Consider the following elements: (Al, Ca, Mg, Cl, P) Part 2: Multiple choice questions (Q10- Q31) Total Marks: 22 (1 Mark each) Q10. How many molecules are present in a drop of propanol, C3H1OH, with a mass of 4.30 a) 4.311019 molecules b) 7.021020 molecules c) 6.311019 molecules d) 9.321019 molecules e) 5.641021 molecules Q11. What is the correct name for HBrO2(aq) ? a) Hydrobromic acid b) Bromic acid c) Bromous acid d) Hypobromous acid e) Perbromic acid Q12. A 35.45mL of HCl solution is needed to react with 2.572g of pure CaCO3. What is the molarity of the HCl solution? CaCO3(s)+2HCl(aq)CaCl2(aq)+H2O(I)+CO2(g) a) 3.33M b) 1.45M c) 4.35M d) 2.90M e) 0.785M Q13. A 45.0mL sample of an element with a density of 1.50g/mL contains 7.001023 atoms. What is the molar mass of this element? a) 83.9g/mol b) 77.4g/mol c) 38.7g/mol d) 95.3g/mol e) 58.1g/mol
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


