Question: Help me answer this 2 question please 7. Use the following information to answer the next question. * 1 point Standard Electrode Potentials VO2+(aq)+2H(aq)++eVO(aq)2++H2O(l)VO2+(aq)+2H(aq)++eV(aq)3++H2O(l)VO2+(aq)+4H(aq)++5eV(s)+2H2O(l)V(aq)3++eV(aq)2+E=+0.999VE=+0.340VE=0.250VE=0.255V Which

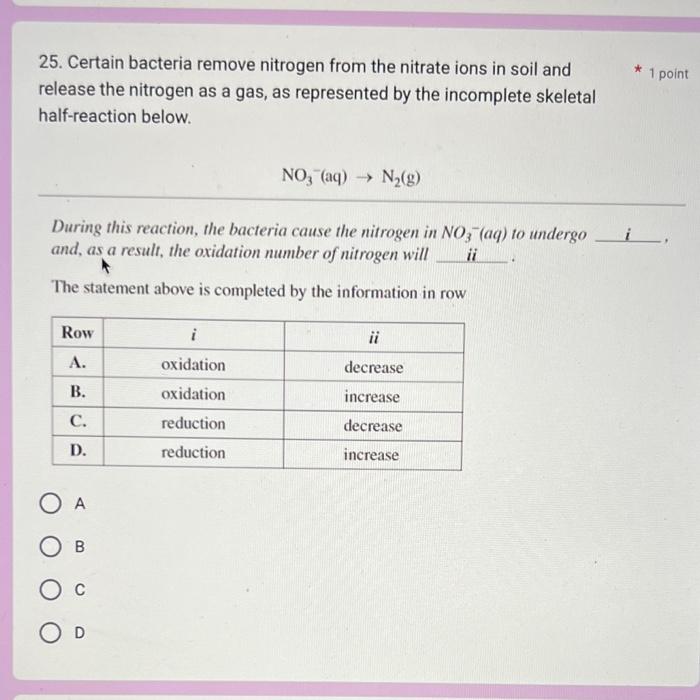
7. Use the following information to answer the next question. * 1 point Standard Electrode Potentials VO2+(aq)+2H(aq)++eVO(aq)2++H2O(l)VO2+(aq)+2H(aq)++eV(aq)3++H2O(l)VO2+(aq)+4H(aq)++5eV(s)+2H2O(l)V(aq)3++eV(aq)2+E=+0.999VE=+0.340VE=0.250VE=0.255V Which of the following substances is the strongest reducing agent? A. V2+(aq) B. V3+(aq) C. VO2+(aq) D. VO(aq)2+ D C B A 25. Certain bacteria remove nitrogen from the nitrate ions in soil and release the nitrogen as a gas, as represented by the incomplete skeletal half-reaction below. NO3(aq)N2(g) During this reaction, the bacteria cause the nitrogen in NO3(aq) to undergo and, as a result, the oxidation number of nitrogen will _ii The statement above is completed by the information in row A B C D
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


