Question: Help me find % KHP and Mean and Standard deviation MOLEST y conc. NaOH = mol NaOH [solve for L NaOH used 1:1 KHP :
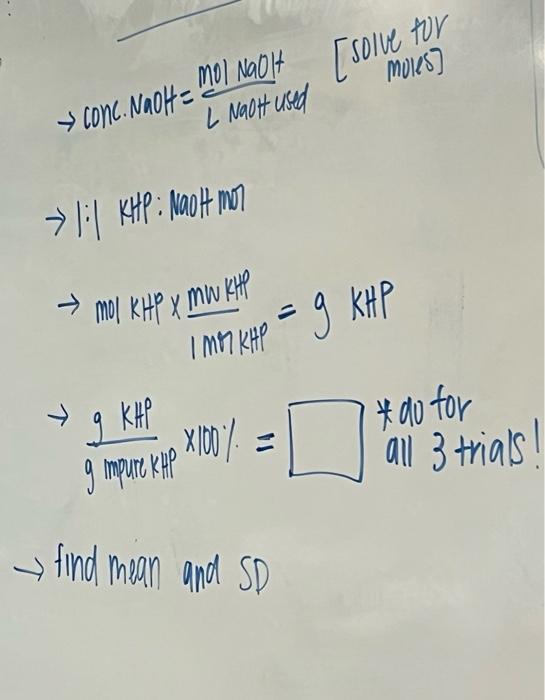
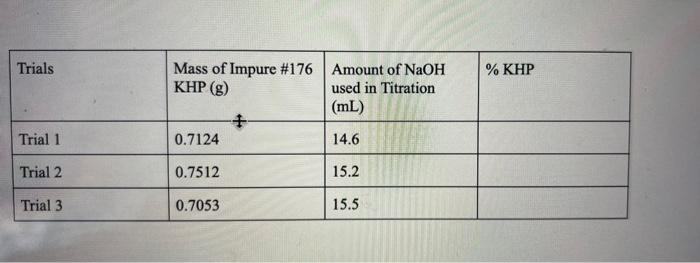
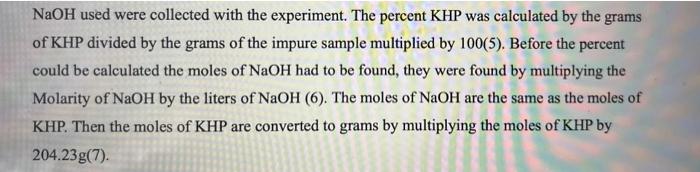
MOLEST y conc. NaOH = mol NaOH [solve for L NaOH used 1:1 KHP : Moot mor mol KHP X MW KHI I MOI KHO = 9 KHP 9 KHP g impure kap X100% #do for x all 3 trials find mean and SD Trials % KHP Mass of Impure #176 Amount of NaOH KHP (g) used in Titration (mL) Trial 1 0.7124 14.6 Trial 2 0.7512 15.2 Trial 3 0.7053 15.5 NaOH used were collected with the experiment. The percent KHP was calculated by the grams of KHP divided by the grams of the impure sample multiplied by 100(5). Before the percent could be calculated the moles of NaOH had to be found, they were found by multiplying the Molarity of NaOH by the liters of NaOH (6). The moles of NaOH are the same as the moles of KHP. Then the moles of KHP are converted to grams by multiplying the moles of KHP by 204.23g(7)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


