Question: help me solve this question step by step plz All chemical reactions are governed by the constraints of equilibrium. A system is said to be
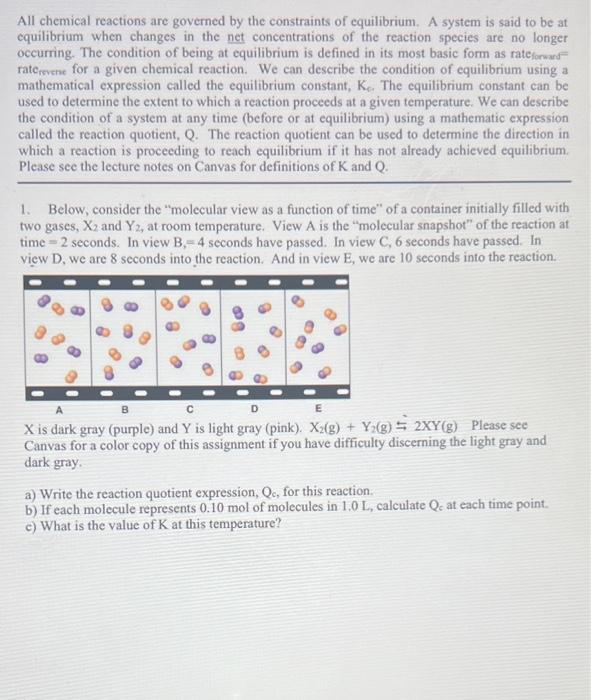
All chemical reactions are governed by the constraints of equilibrium. A system is said to be at equilibrium when changes in the net concentrations of the reaction species are no longer occurring. The condition of being at equilibrium is defined in its most basic form as rateforwars= rate ervene for a given chemical reaction. We can describe the condition of equilibrium using a mathematical expression called the equilibrium constant, Kc. The equilibrium constant can be used to determine the extent to which a reaction proceeds at a given temperature. We can describe the condition of a system at any time (before or at equilibrium) using a mathematic expression called the reaction quotient, Q. The reaction quotient can be used to determine the direction in which a reaction is proceeding to reach equilibrium if it has not already achieved equilibrium. Please see the lecture notes on Canvas for definitions of K and Q. 1. Below, consider the "molecular view as a function of time" of a container initially filled with two gases, X2 and Y2, at room temperature. View A is the "molecular snapshot" of the reaction at time =2 seconds. In view B,=4 seconds have passed. In view C,6 seconds have passed. In view D, we are 8 seconds into the reaction. And in view E, we are 10 seconds into the reaction. X is dark gray (purple) and Y is light gray (pink). X2(g)+Y2(g)2XY(g) Please see Canvas for a color copy of this assignment if you have difficulty discerning the light gray and dark gray. a) Write the reaction quotient expression, Qc, for this reaction. b) If each molecule represents 0.10mol of molecules in 1.0L, calculate Qc at each time point. c) What is the value of K at this temperature
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


