Question: Help on these two 8. At 500.0 K, one mole of gaseous ONCI is placed in a one-liter container. At equilibrium it is 9.0% dissociated
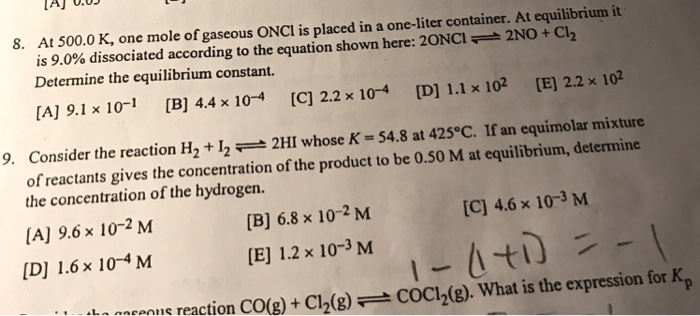
8. At 500.0 K, one mole of gaseous ONCI is placed in a one-liter container. At equilibrium it is 9.0% dissociated according to the equation shown here: 20NCI 2NO + Cl Determine the equilibrium constant. [A] 9.1 x 10-1 [B] 4.4 x 10-4 [C] 2.2 x 10-4 [D] 1.1 x 10 [E] 2.2 x 102 9. Consider the reaction H + 122HI whose K = 54.8 at 425C. If an equimolar mixture of reactants gives the concentration of the product to be 0.50 M at equilibrium, determine the concentration of the hydrogen. [A] 9.6 10-2 M [D] 1.6 x 10-4 M [C] 4.6 x 10-3 M 1-(1+1) = -1 ons reaction CO(g) + Cl(g) COC1(g). What is the expression for Kp [B] 6.8 x 10-2 M [E] 1.2 x 10-3 M
Step by Step Solution
3.46 Rating (146 Votes )
There are 3 Steps involved in it
8 20 HC Intial Im 1X H I 9 2NOC1 at equilbirium 091 9x g... View full answer

Get step-by-step solutions from verified subject matter experts


