Question: help on these two asap. first pic is question 1 and pics 2&3 are question 2 The structure below has an unusually large dipole. Draw
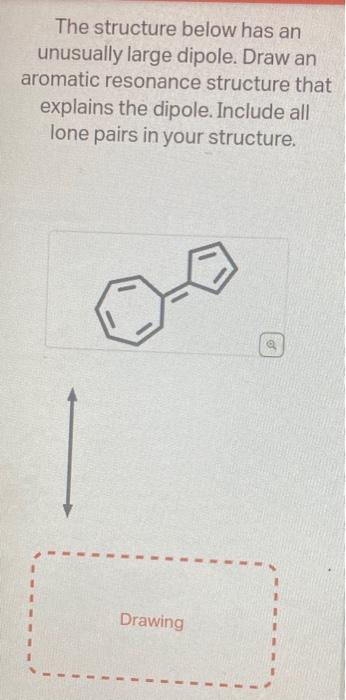
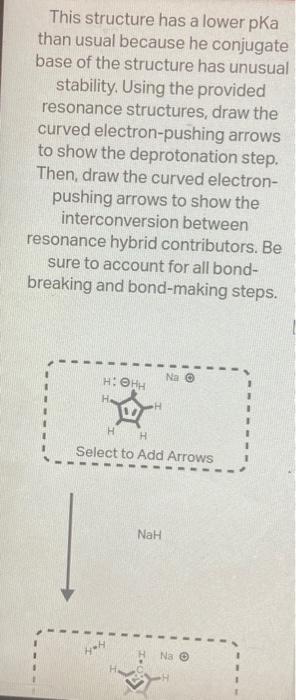
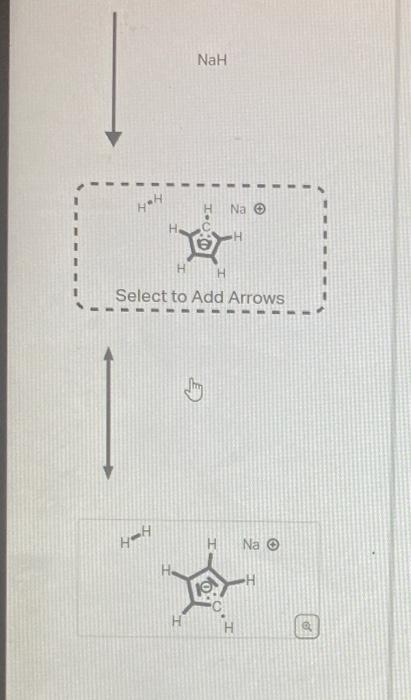
The structure below has an unusually large dipole. Draw an aromatic resonance structure that explains the dipole. Include all lone pairs in your structure. This structure has a lower pKa than usual because he conjugate base of the structure has unusual stability. Using the provided resonance structures, draw the curved electron-pushing arrows to show the deprotonation step. Then, draw the curved electronpushing arrows to show the interconversion between resonance hybrid contributors. Be sure to account for all bondbreaking and bond-making steps. NaH Select to Add Arrows
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


