Question: help please Consider the following electron configurations to answer the questions that follow: 0 152 282 2p 3s1 (ii) 152 2s 2p 3s2 (ii) 152
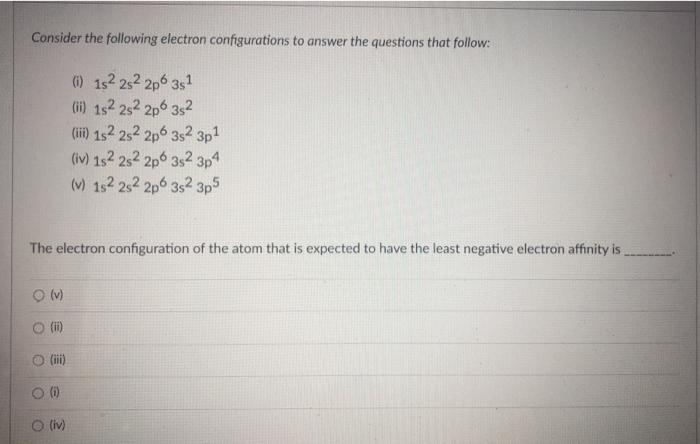
Consider the following electron configurations to answer the questions that follow: 0 152 282 2p 3s1 (ii) 152 2s 2p 3s2 (ii) 152 2s22p6352 3p1 (iv) 152 2s22p6 352 3p4 () 152 282 2p 3s 3p5 The electron configuration of the atom that is expected to have the least negative electron affinity is (v) O (1) (iii) (iv)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


