Question: help please i dont understand how to do this Consider the reaction. MnS(s)+2HCl(aq)MnCl2(aq)+H2S(g) If the small amount of MnS is combined with the large amount

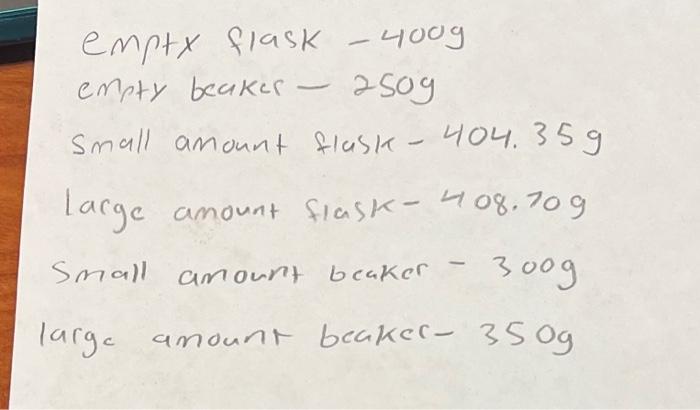
Consider the reaction. MnS(s)+2HCl(aq)MnCl2(aq)+H2S(g) If the small amount of MnS is combined with the large amount of HCl, what is the mass of the products? The mass of any excess reactants will also be included in this total. mass: empty flask - 4009 empty beakes - 2509 small amount flask - 404.35g lacge amount fiask - 408.709 Small amount beaker - 300g arge amount beakec- 350g
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


