Question: help please Model 3b: Mixing the Solutions Together: What Happens? At 25C, the contents of Beakers A and B are combined into Beaker C, which
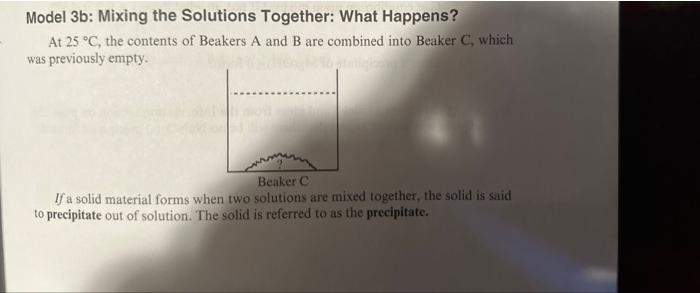
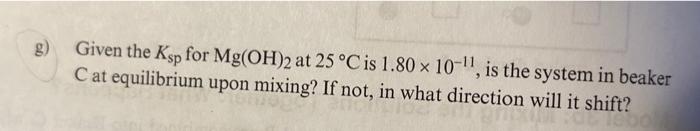
Model 3b: Mixing the Solutions Together: What Happens? At 25C, the contents of Beakers A and B are combined into Beaker C, which was previously empty. If a solid material forms when two solutions are mixed together, the solid is said to precipitate out of solution. The solid is referred to as the precipitate. g) Given the Ksp for Mg(OH)2 at 25C is 1.801011, is the system in beaker C at equilibrium upon mixing? If not, in what direction will it shift
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


