Question: Help pls & thank you ! I tried doing it based on an example I saw but did not grasp it entirely. How many grams
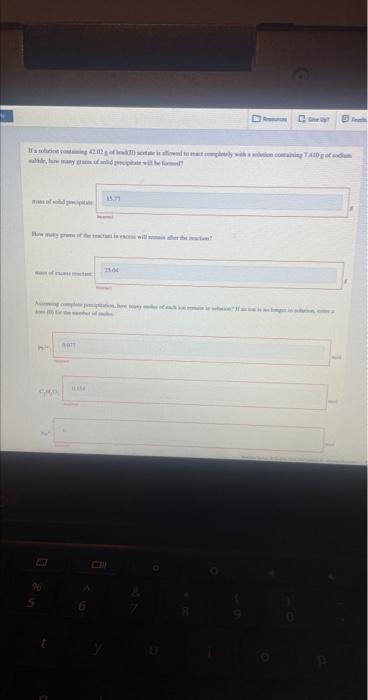
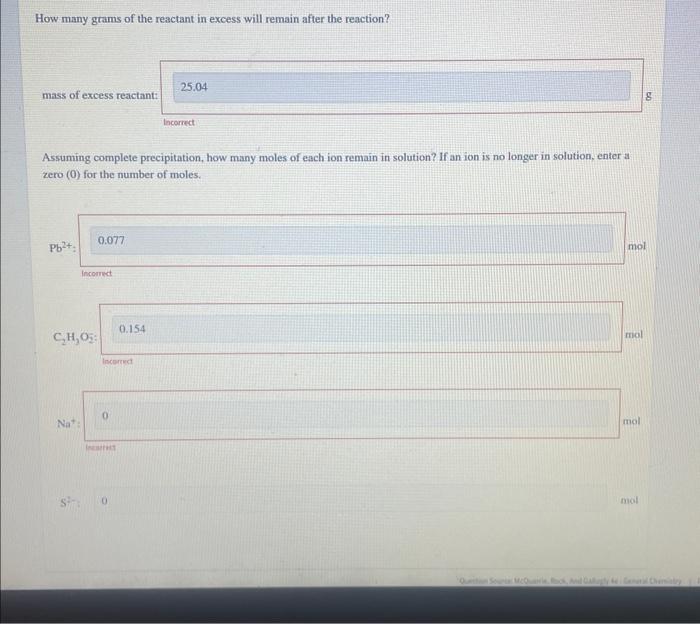
How many grams of the reactant in excess will remain after the reaction? mass of excess reactant: tacarrect Assuming complete precipitation, how many moles of each ion remain in solution? If an ion is no longer in solution, enter a zero (0) for the number of moles. Incomed
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


