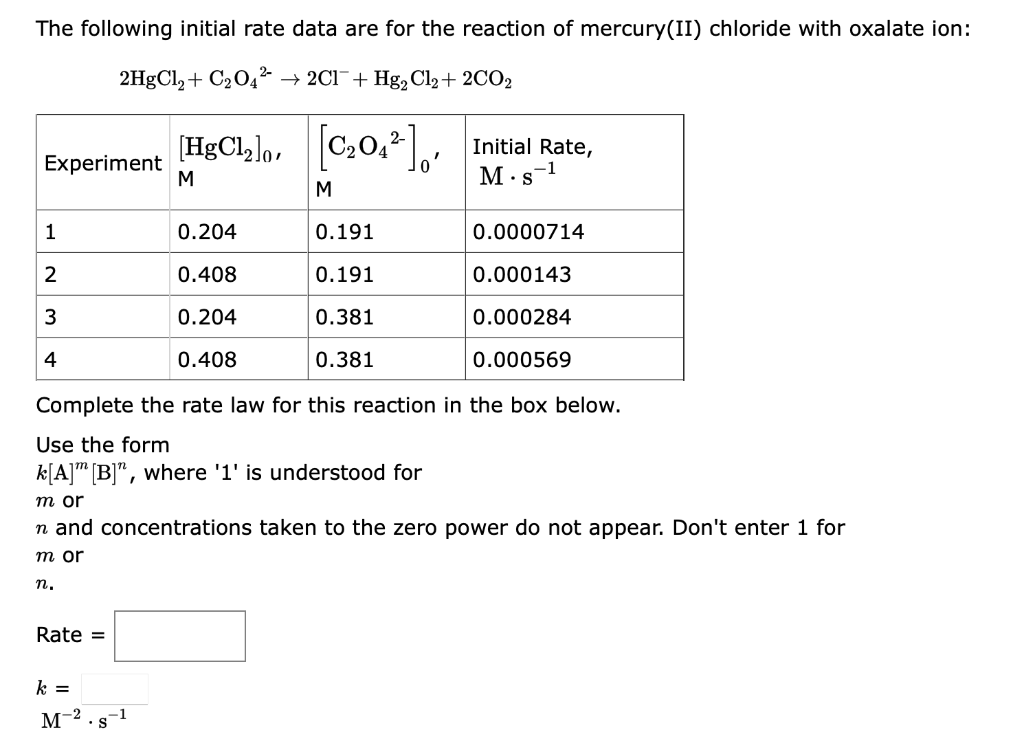
Question: HELP PLS! The following initial rate data are for the reaction of mercury(II) chloride with oxalate ion: 2HgCl2+C2O422Cl+Hg2Cl2+2CO2 Complete the rate law for this reaction
 HELP PLS!
HELP PLS!
The following initial rate data are for the reaction of mercury(II) chloride with oxalate ion: 2HgCl2+C2O422Cl+Hg2Cl2+2CO2 Complete the rate law for this reaction in the box below. Use the form k[A]m[B]n, where ' 1 ' is understood for m or n and concentrations taken to the zero power do not appear. Don't enter 1 for m or n. Rate = k= M2s1
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


