Question: help plz For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they will,
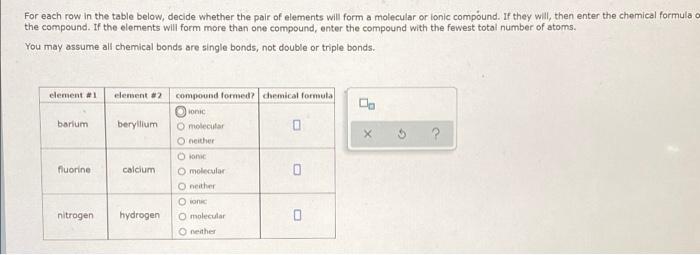
For each row in the table below, decide whether the pair of elements will form a molecular or ionic compound. If they will, then enter the chemical formula o the compound. If the elements will form more than one compound, enter the compound with the fewest total number of atoms. You may assume all chemical bonds are single bonds, not double or triple bonds. element #1 element #2 barium beryllium ? compound formed chemical formula Oonic O molecular 0 neither O molecular neither On O molecular 0 neither fluorine calcium nitrogen hydrogen
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


