Question: help! reaction engineering. not sure how to get the selectivity. The data seems off to me too. I'll see if I can track down more
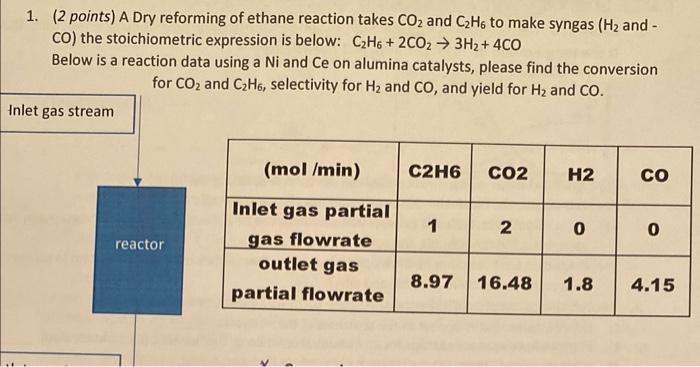
1. (2 points) A Dry reforming of ethane reaction takes CO2 and C2H6 to make syngas (H2 and CO) the stoichiometric expression is below: C2H6+2CO23H2+4CO Below is a reaction data using a Ni and Ce on alumina catalysts, please find the conversion for CO2 and C2H6, selectivity for H2 and CO, and yield for H2 and CO
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


