Question: help Suppose a new element with an average atomic mass of 22.929u is discovered on Mars. This element is isotopes. The most abundant isotope has
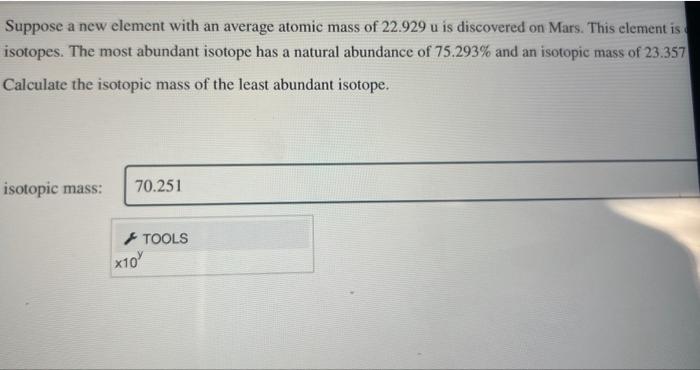
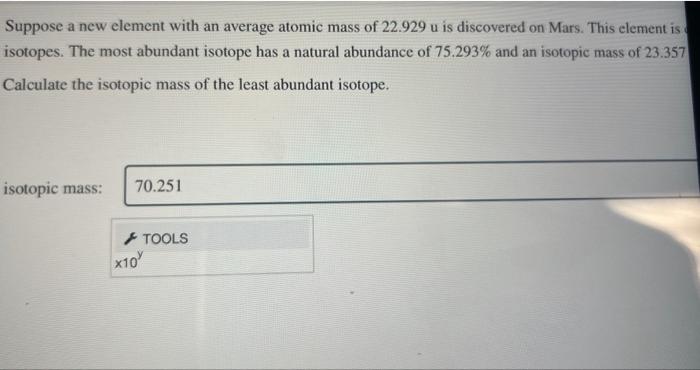
Suppose a new element with an average atomic mass of 22.929u is discovered on Mars. This element is isotopes. The most abundant isotope has a natural abundance of 75.293% and an isotopic mass of 23.357 Calculate the isotopic mass of the least abundant isotope. isotopic mass: Suppose a new element with an average atomic mass of 22.929u is discovered on Mars. This element is isotopes. The most abundant isotope has a natural abundance of 75.293% and an isotopic mass of 23.357 Calculate the isotopic mass of the least abundant isotope. isotopic mass
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


