Question: HELP THIS? Watch KCV: The Mole Concept, Lonverting between the Number of Moles and Number of Atoms; Read Section 2.9. You can click on the
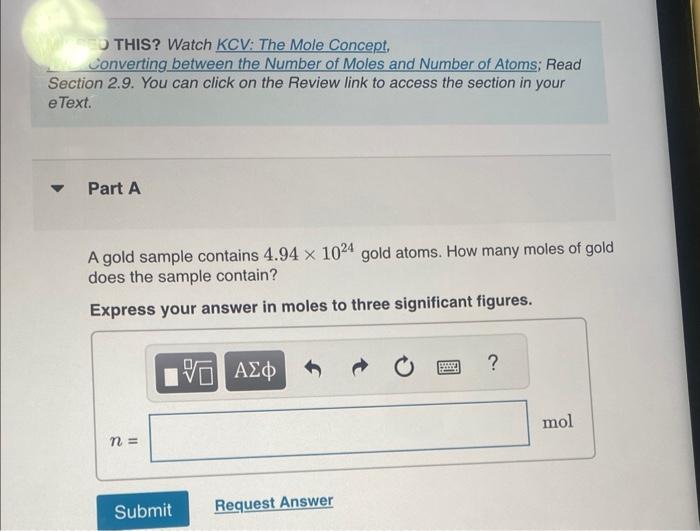
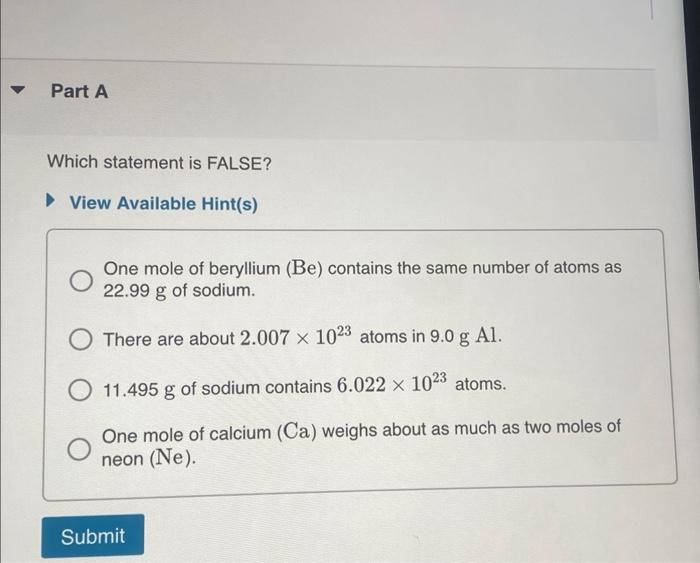
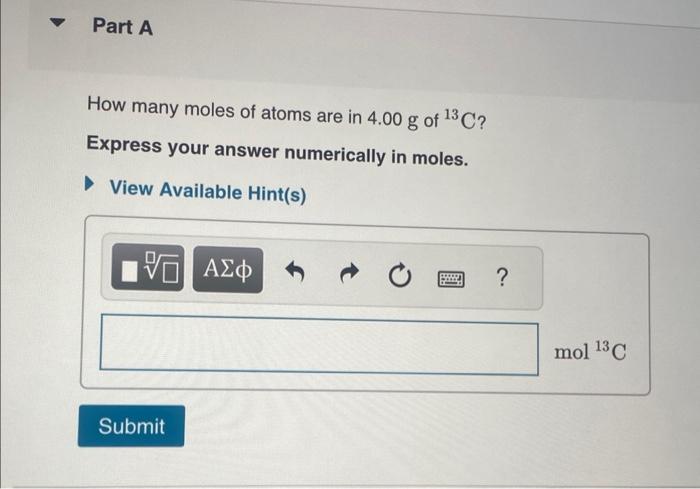
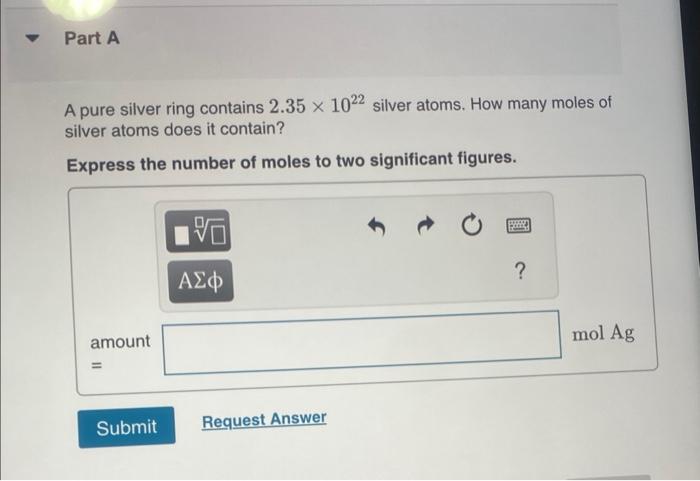
THIS? Watch KCV: The Mole Concept, Lonverting between the Number of Moles and Number of Atoms; Read Section 2.9. You can click on the Review link to access the section in your e Text. Part A A gold sample contains 4.941024 gold atoms. How many moles of gold does the sample contain? Express your answer in moles to three significant figures. One mole of beryllium ( Be) contains the same number of atoms as 22.99g of sodium. There are about 2.0071023 atoms in 9.0gAl. 11.495g of sodium contains 6.0221023 atoms. One mole of calcium (Ca) weighs about as much as two moles of neon (Ne). How many moles of atoms are in 4.00g of 13C ? Express your answer numerically in moles. A pure silver ring contains 2.351022 silver atoms. How many moles of silver atoms does it contain? Express the number of moles to two significant figures. How many sulfur atoms are there in 5.00mol of sulfur? Express your answer using three significant figures
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


